Table 3.
Optimization of 5-styryl-oxathiazolone inhibitors with regard to solubility.
| Entry | Structure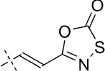
|
Cmpd | Method, Yield | Mtb proteasome IC50 [nM] | Human proteasome IC50 [nM] | Solubility [μM] | Stability [%][a] |
|---|---|---|---|---|---|---|---|
| 1 | 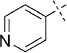 |
49 | D; B, 7 %[b] | 2750 | 77 000 | 84.7 | 91.6 |
| 2 | 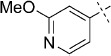 |
50 | D; A, 39 %[b] | 15 000 | 29 500 | 2.1 | 97.7 |
| 3 | 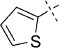 |
51 | C, 46 %; B, 45 % | 2100 | 18 500 | 22.6 | 97.6 |
| 4 | 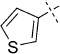 |
52 | C, 75 %; B, 53 % | 1150 | 11 300 | 19.7 | 99.1 |
| 5 | 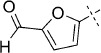 |
53 | C; B, 5 %[b] | 735 | 3300 | 56.1 | 88.9 |
| 6 | 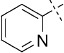 |
54 | E; A, 3 %[b] | 2350 | >10 000[c] | 64.6 | n.d. |
| 7 | 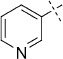 |
55 | E; A, 15 %[b] | 1350 | >10 000[c] | 70.6 | n.d. |
| 8 |  |
56 | E; B, 4 %[b] | 2100 | >10 000[c] | 2.4 | n.d. |
| 9 |  |
57 | –, 31 %;[d] B, 12 % | 1200 | 5000 | 4.1 | n.d. |
General method for the synthesis of 2-substituted acrylamides. Method C reagents and conditions: starting aryl iodide (1.0 equiv), acrylamide (2.0 equiv), Pd(OAc)2 (0.05 equiv), tri-tert-butylphosphonium tetrafluoroborate (0.10 equiv), Et3N (3.0 equiv), CH3CN (4.0 mL mmol−1), 120 °C MW, 15 min. Method D reagents and conditions:. starting aryl iodide (1.0 equiv), acrylamide (2.0 equiv) and Pd(OAc)2 (0.05 equiv), Et3N (3.0 equiv), CH3CN (4.0 mL mmol−1), 120 °C MW, 15 min. Method E reagents and conditions: starting aryl bromide (1.0 equiv), acrylamide (1.5 equiv) and trans-bis(acetato)bis[o-(di-o-tolylphosphino)benzyl]dipalladium(II) (0.05 equiv), NaOAc (3.0 equiv), DMF (4.0 mL mmol−1), 140 °C MW, 15 min. [a] Chemical stability in PBS pH 7.4 at 25 °C; as % remaining after 24 h. [b] Isolated yield over two steps. [c] The compound interferes with the assay at 10 μm, therefore it was not possible to measure human proteasome inhibition. [d] Reagents and conditions: 1) (2E)-3-(1H-Indolyl-3-yl)acrylic acid (2.0 mmol), 1,1′-carbonyldiimidazole (2.0 mmol), DMF (10 mL), rt, 30 min, 2) NH4HCO3 (4.0 mmol), rt, o/n.
