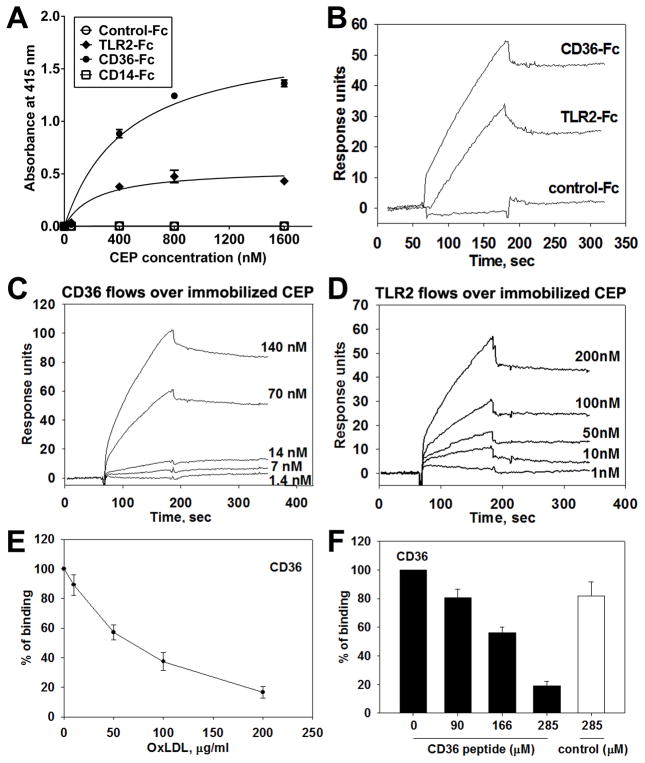
Figure 4. Direct binding between CEP and CD36 or TLR2.
A. ELISA-based binding assay using various concentrations of CEP-BSA (0 to 1600 nM) and Fc-conjugated chimeric proteins, such as TLR2-Fc, CD36-Fc, CD14-Fc. Binding to Fc-only served as a negative control (control-Fc). The absorbance values at 415 nm are shown. Values obtained using BSA-alone (as a coating substrate) were subtracted. B–D. Surface plasmon resonance (SPR) analysis of the binding between CEP and CD36 (B, C, E, and F), CEP and TLR2 (B and D). CD36-Fc, TLR2-Fc, and Fc-only were immobilized on CM5 chip and 2.5 μM CEP-HSA in HEPES buffer was flowed over the chip (B). CEP-HSA was immobilized on a CM5 chip and various concentrations of CD36-Fc (1.4 to 140 nM) (C) or TLR2-Fc (1 to 200 nM) (D) were flowed over the chip. Note that CEP demonstrates direct binding to CD36 and, to a lesser extent, TLR2. E and F. SPR analyses for CEP binding site on CD36. 70 nM of CD36-Fc were preincubated with various concentrations of copper oxidized LDL (10 to 200 μg/ml) and flowed over immobilized CEP-HAS. CEP binding to CD36 in the absence of OxLDL was assigned a value of 100% (E). Various concentrations of CD36 peptide (160SLINKSKSSMF170) or scrambled peptide (SIKVNLSQMKILNSI) were preincubated with 10 μM of CEP-HSA and flowed over immobilized CD36-Fc. CEP binding to CD36 in the absence of peptide was assigned a value of 100% (F).