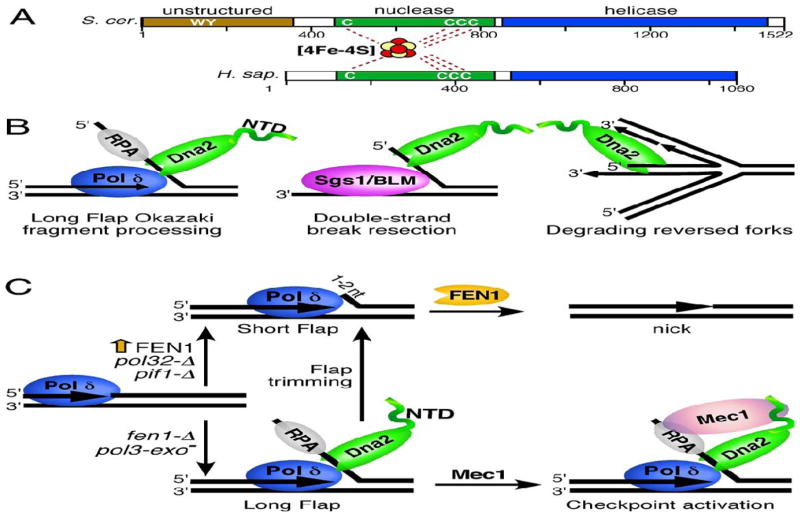
Figure 1. The structure and functions of Dna2.
(A) A schematic representation of the domain structure of S. cerevisiae and human Dna2 nuclease/helicase. The nuclease and helicase domains are depicted in green and blue, respectively. The N-terminal unstructured domain of yeast Dna2 is in light brown. The N-terminal domain of human Dna2 is shorter than that of its yeast counterpart and does not contain an appreciable unstructured region. The cysteines that coordinate the iron-sulfur domain as well as the Trp and Tyr that mediate Mec1ATR activation are indicated. (B) Some of the functions of Dna2 in maintenance of nuclear genome stability. Left panel, Dna2 mediates processing of long flaps during Okazaki fragment maturation. Middle panel, Dna2 works in conjunction with Sgs1/BLM helicase in a pathway of DSB end resection. Right panel, Dna2 prevents reversal of replication forks. Additional details are found in the text. (C) The role of Dna2 during Okazaki fragment maturation. On the lagging strand, displacement synthesis by Pol δ generates flaps of 1–2nt that are cleaved by FEN1 to create a ligatable nick (short flap pathway, top of figure). Some flaps escape FEN1 cleavage and grow long enough to bind RPA (long flap pathway, bottom of figure). These long flaps require initial cleavage by Dna2 to allow final processing by FEN1 (flap trimming). Conditions or mutations that promote generation of long flaps (Pol δ exonuclease deficiency or fen1-Δ) are dependent on a functional Dna2, whereas FEN1 overexpression, deletion of the POL32 subunit of Pol δ or deletion of PIF1 promote the short flap pathway and show less dependence on Dna2 [27]. Dna2 bound to long flaps may trigger the checkpoint by activating Mec1ATR.