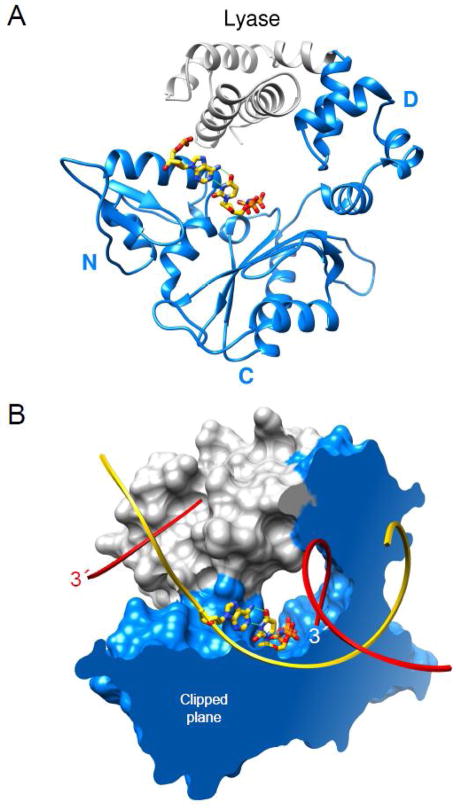
Fig. 2.
Domain/subdomain organization of human DNA polymerase β. (A) A ribbon representation of pol β illustrating the polymerase (blue) and amino-terminal lyase (gray) domains. The polymerase domain is composed of three subdomains: DNA-binding, D; catalytic, C; and nascent base pair interacting, N. These correspond to the thumb, palm, and fingers subdomains of DNA polymerases, respectively, that utilize an architectural analogy to a right hand. The nascent base pair (yellow carbons) is illustrated in a stick representation while the remaining DNA is omitted for clarity. (B) The molecular surface of pol β bound to single-nucleotide gapped DNA exhibits a global doughnut-like structure where the lyase domain interacts with the N–subdomain of the polymerase domain. The viewpoint is the same as panel A. The molecular surface is clipped through the nascent base pair (illustrated in a stick representation) binding pocket. The backbone of the template and broken strands are shown in a ribbon representation; yellow and red, respectively. The 3′-ends of the gapped strand are indicated (the 3′-end label of the primer strand near the polymerase active site is white).