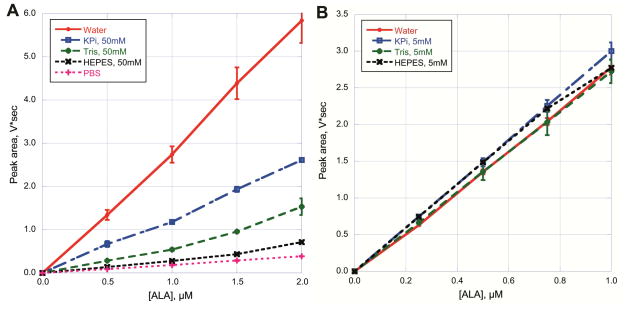
Figure 1.
Influence of buffers on derivatization of ALA. The area under the curve (V*sec) is plotted against ALA concentration in μM. (A) Buffers at a 50 mM concentration are optimal for the initial ALAS reaction, potassium phosphate (KPi). Tris, HEPES and phosphate buffered saline (PBS) were titrated to pH 7.4 and tested to determine if there was any effect on the ability to convert ALA (0.5, 1.0, 1.5 and 2.0 μM) to 2,6-diacetyl-1,5-dimethyl-7-(2-carboxyethyl)-3-pyrrolizine. (B) Assay mixtures from (A) were diluted 10X with water and then derivatized.