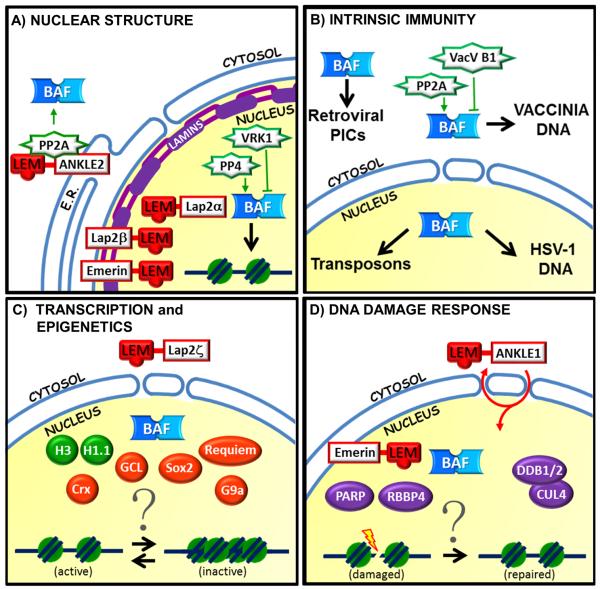
Figure 1.
Overview of BAF-associated proteins in different functional pathways. (A) Nuclear structure. BAF interacts dynamically with LEM-domain proteins, lamins and chromatin at the inner nuclear membrane and in the nucleoplasm. BAF also functions dynamically in the cytoplasm. BAF binding to key partners including DNA and LEM-domain proteins is inhibited by phosphorylation (by VRK1) and promoted by dephosphorylation (by PP2A and PP4). (B) Intrinsic Immunity: In the cytoplasm BAF intercepts and fights certain viruses by binding their DNA (the poxvirus vaccinia), but is acquired and exploited to protect retroviral DNA in preintegration complexes. BAF defense against vaccinia infection may be supported by PP2A-dependent dephosphorylation of BAF, competing against vaccinia B1 kinase-dependent inhibitory phosphorylation of BAF. Within the nucleus, BAF inhibits the replication and transcription of the DNA virus HSV-1 as part of the host innate immune defense. Remarkably BAF binds and protects endogenous transposons (mobile genetic elements), a role with implications for the evolution of eukaryotic genomes. (C) Transcription and Epigenetics: BAF influences transcription, and associates with proteins known to regulate transcription including LEM-domain proteins (emerin, MAN1, Lap2β not shown; Lap2ζ shown), transcription factors (Sox2, Crx, GCL, Requiem), specific histones (H3, H1.1) and histone modifiers (G9a). However the mechanisms of BAF involvement in chromatin regulation remain unknown. (D) DNA Damage Response. BAF is needed for a robust DNA damage response, and interacts with proteins that respond to or mediate the DNA damage response including Ankle1/LEM3, emerin, RBBP4, DDR1/2, CUL4 and PARP. However the mechanisms of BAF involvement are open questions.