Abstract
Background
Ultrasonic nebulization (UN) and oxygen-driven nebulization (ON), two commonly used modalities for nebulization inhalation, are not ideally suitable for patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD).
Methods
A total of 91 patients with AECOPD were randomized to three groups given different nebulization modalities: ON, UN, and ultrasonic nebulization with warming and oxygen (UNWO). The sputum clearance, lung function, changes in physiological measures such as peripheral oxygen saturation (SpO2) and tolerance to these nebulization modalities were recorded and compared among the three groups.
Results
The time to the first expectoration was shorter and the sputum volume was larger after UN and UNWO than after ON (both P<0.01). Compared with pre-nebulization, SpO2 significantly increased (P<0.01) and the dyspnea decreased significantly (P<0.05) after UNWO. The SpO2 and dyspnea post-UNWO were significantly better than those post-UN (P<0.01, P<0.05), but not statistically different from those post-ON (both P>0.05). UNWO demonstrated significantly greater comfort and longer duration of nebulization than UN (P<0.01, P<0.05), but no significant differences in these respects from ON (both P>0.05). Forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and peak expiratory flow (PEF) decreased significantly after UNWO (P<0.05, P<0.01, and P<0.01, respectively).
Conclusions
UNWO may promote expectoration of sputum with fewer adverse reactions and a higher level of comfort than simple UN and ON. Therefore, it can be used as an adjuvant therapy for AECOPD patients.
Keywords: Oxygen-driven nebulization (ON), ultrasonic nebulization (UN), ultrasonic nebulization with warming and oxygen (UNWO), sputum expectoration, chronic obstructive pulmonary disease (COPD), acute exacerbation of chronic obstructive pulmonary disease (AECOPD)
Introduction
Nebulized inhalation is a common procedure given to patients with respiratory diseases that helps moisten and dilute viscous airway secretions, and thereby facilitates their clearance. Addition of therapeutic agents (such as antibiotics and bronchodilators) to the nebulizing solution can further lead to direct, local effects against airway inflammation, obstruction or constriction (1).
Most currently available nebulizers use oxygen [oxygen-driven nebulization (ON)] or ultrasound [ultrasonic nebulization (UN)] to break up medical solutions into tiny droplets that can be directly inhaled from the mouthpiece of the device. However, the high-speed oxygen in ON may cause hypercapnia in acute exacerbation of chronic obstructive pulmonary disease (AECOPD) complicated by type II respiratory failure (1,2). On the other hand, UN does not contain augmented oxygen, and among certain patients whose condition needs so, may adversely lead to hypoxemia and bronchial constriction (3-5). For these patients, nasal oxygen has been shown to improve blood oxygen tension and relieve hypoxic symptoms during an UN (6).
Several studies have shown that breathing vapor-saturated air at near body temperature (37 °C, 100% relative humidity) is associated with optimal gas-liquid dynamics and may help effective mucociliary clearance in the airway (7-9); whereas below a temperature of 30 °C, the airway mucociliary motions may become weakened and hence less protective of the human body (10). In addition, the high-speed gas flow in jet nebulization (as in ON) may cool down the solution in the liquid reservoir (11) by as much as 7 to 10 °C (12,13). Our pilot study also indicated that a 20-min nebulization of 0.9% normal saline at room-temperature subsequently gave rise to a reduction in aerosol temperature by 5 °C with ON (EM06-003, eMedical®, Excellentcare Medical Ltd., London, UK), compared with a 1 °C increase with UN (Yuehua Medical Instrument Co. Ltd., Shantou, China). Therefore, aerosol generated from UN or ON under the room temperature (20 °C), such as is used in most clinical settings, may not be sufficiently warm to optimize airway mucociliary movements.
We hypothesized that a novel modality of nebulization, UN with additional warming and oxygen augmentation (UNWO), may be more effective in facilitating sputum expectoration, and would be associated with less adverse effects (hypoxemia and bronchoconstriction), as compared with ON and classical UN. Here we presented a validation study in patients with AECOPD.
Methods
Patient recruitment
Between December 2010 and November 2011, a cohort of 93 hospitalized patients with AECOPD diagnosed according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines 2009 (14) at the First Affiliated Hospital of Guangzhou Medical University, were recruited for the present study. The exclusion criteria were: (I) concomitant bronchial asthma or hemoptysis; (II) a history of chest surgery or active bleeding during the previous 3 months; (III) inability to complete a pulmonary function test (PFT) due to certain conditions (pulmonary bullae, dyspnea, pulmonary embolism necessitating bed rest, or coronary heart disease); and (IV) infectious diseases, mental disorders, severe cognitive impairment, or other conditions that may prevent the patient from cooperating with the investigators.
This study was a clinical trial registered with the Chinese Clinical Trial Registry (http://www.chictr.org/cn/proj/show.aspx?proj=5131, No.: ChiCTR-TRC-13003330).
Ethics statement
The study protocol was approved by the Ethics Committee of First Affiliated Hospital, Guangzhou Medical University (Number of Approval: GYFYY-2010-35). All patients gave written informed consent prior to entering the study.
Study design and treatment protocol
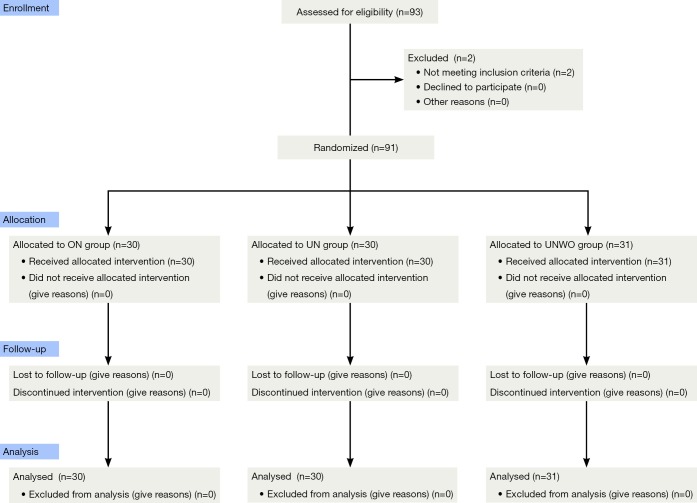
This was a double-blind randomized controlled trial. Decision on the need for nebulized inhalation in these subjects was confirmed by their physicians in charge. Per exclusion criteria, 2 male patients were excluded because of concomitant bronchial asthma, and 91 were included in this study. Based on a computer-generated random-digit table, the eligible subjects were assigned to receive ON group (n=30), classical UN group (n=30), or UNWO group (n=31) (Figure 1). Zeguang Zheng generated the random allocation sequence, and enrolled participants, and Qiaoling Luo assigned participants to interventions.
Figure 1.
Consort 2010 flow diagram of study.
About 0.9% normal saline was used for 20-min nebulized inhalation via a wall-mounted mouthpiece in all three groups. To achieve double-blindness, the aerosols were generated by nebulizers in the generator room immediately next to the treatment room they were inhaled through conveying ducts.
During the nebulization, the patients were instructed to take a sitting or semi-recumbent position which may facilitate slow and deep breaths, so that the aerosol could be inhaled into the airway as deeply and as much as possible. We employed a disposable medical nebulizer (EM06-003, eMedical®, Excellentcare Medical Ltd., London, UK) for ON (oxygen flow rate =6 L/min) and a WH-802 ultrasonic nebulizer (Yuehua Medical Instrument Co. Ltd., Shantou, China) for classical UN (small air flow, aerosol output =2 mL/min). For UNWO, the same type of ultrasonic nebulizer as in UN was used, while a patented heater and temperature regulating set were added to the UN system (Figure 2). The added set has been validated and approved by China Intellectual Property Bureau (License number of utility model: 201220412530.3; License number of patented invention: 201110193601.5), and was intended to regulate the aerosol temperature automatically according to manually set temperatures. In addition, an oxygen tube connected the UN to oxygen source. In this setting, the aerosol produced by UNWO contains a suitable temperature (about 35 °C) and a moderate oxygen flow rate (2-3 L/min).
Figure 2.
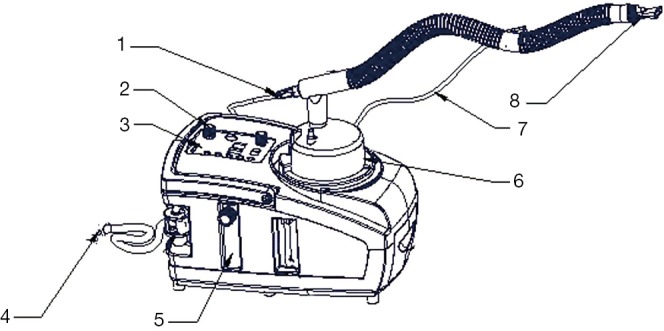
The device for UNWO. Aerosol generated by ultrasonic nebulizer was directed through a warming and oxygen-augmenting unit to reach a calibrated temperature of 32-35 °C at the mouthpiece. 1, heat connector; 2, power switch; 3, control panel; 4, oxygen tube (connected to oxygen source); 5, oxygen flow meter; 6, liquid reservoir; 7, temperature probe; 8, mouthpiece. UNWO, ultrasonic nebulization with warming and oxygen.
Clinical and experimental measures
At baseline, the patients were instructed to cough and expectorate voluntarily for several attempts until an adequate clearance of their airway secretions was perceived. Then the patients were observed for 30 min to be recorded for frequency of cough (FoC), time to first expectoration (T2E), and total sputum volume (30mSV) during this period. At the end of the 30-min observation, a PFT (baseline spirometry) was performed to measure the forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and peak expiratory flow (PEF) in all patients. Following baseline spirometry, the patients received inhalation of 400 µg salbutamol and were subjected to a 20-min rest.
Next, the patients were once again required to cough and expectorate voluntarily until an adequate clearance of their airway secretions was perceived, and then given nebulized inhalation of normal saline for 20 min. An observation was made during the interval from start of the 20-min nebulizing procedure to 30 min later for their cough and expectoration (including FoC, T2E and 30mSV). Peripheral oxygen saturation (SpO2), heart rate (HR), respiratory rate (RR), dyspnea, and chest tightness were also measured in the patients before and at every 5 min throughout the nebulization. At 30 min from start of the 20-min nebulization, the patients underwent a second spirometry and their perceived comfort about the nebulization (Figure 3). In addition, we measured the volume of normal saline in the liquid reservoir (with a standard measuring cylinder) as well as the mouthpiece aerosol temperature (with an YL-D-6 electronic thermometer, Yilian Control Temperature Apparatus Factory, Shanghai, China) at the beginning and the end of nebulization. The mean consumption of normal saline over the 20-min nebulizing procedure was calculated and defined as the nebulization rate (mL/min) of each nebulization modality (ON, UN and UNWO).
Figure 3.
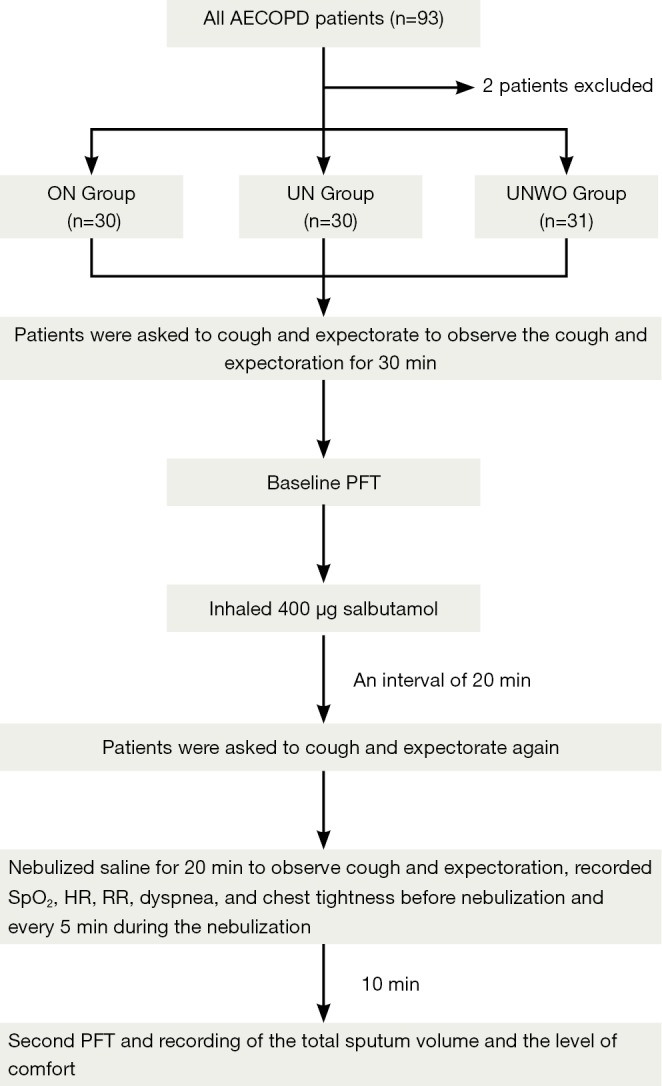
The study protocol.
Dyspnea was rated by the modified Borg Scale (mBs) (15). Chest tightness and patient comfort about the nebulization were determined by using a visual analog scale (VAS) ranging from 0 to 10 (16), where 0 indicated “very uncomfortable” or “worst chest tightness”, and 10 indicated “very comfortable” or “no chest tightness”. The T2E was evaluated with a scoring system as follows: 0-5 min =1, 6-10 min =2, 11-15 min =3, 16-20 min =4, 21-25 min =5, 26-30 min =6, and >30 min =7. As such, a higher score indicated longer T2E.
In a separate in vitro experiment, the mean distribution of particle size was obtained by repeated measurement of the aerosol immediately at the end of 20-min UN, ON and UNWO for 5 times with a P-02 laser particle size analyzer (OMEC Technology Co. Ltd., Guangdong, China).
Unscheduled termination of nebulization
Before the scheduled 20-min duration was completed, the nebulization would be terminated if the patient had any of: (I) progressive difficulty breathing and a level of SpO2 <85%, or a >10% reduction in SpO2; (II) an intense cough caused by the aerosol; (III) intolerable chest tightness; or (IV) disordered consciousness. For each patient, we recorded the actual duration of nebulizing procedure, and if any, the reason for his or her unscheduled termination of the nebulization.
Power calculation
A sample size of approximately 16 patients per group is enough to provide 80% power to detect the treatment effect equal to 1 standard deviation (SD) of FEV1, with the use of a two-sided test (α=0.05). The sample size for each group used in this study was nearly two times of that, getting higher power of test (more than 95% when two-sided α=0.05, more than 90% when two-sided α=0.01).
Statistical analysis
The data were processed by using the Statistical Package for the Social Sciences (Version 13.0, SPSS Inc., and Chicago, IL, USA). All data were subjected to normality test if applicable. The numerical data were presented as mean ± SD. Paired t-test was used to compare the numerical data obtained at baseline, immediately prior to nebulization and post-nebulization. One-way analysis of variance (ANOVA) or analysis of covariance (ANCOVA) was used for inter-group comparison. Comparisons of dynamic observation data among groups were performed using repeated-measure ANOVA. For non-normally distributed data and categorical data, pairwise comparisons were performed using the least significant difference (LSD) test or Mann-Whitney U-test. A level of P<0.05 was considered statistically significant.
Results
Patient demographics and baseline characteristics
Of the 91 patients included in this study, the male-to-female ratio was 83 to 8, and the mean age was 71.6±8.7 years. There were no significant differences in patient demographics or baseline characteristics between groups (Table 1). The three groups of patients were also comparable concerning the GOLD staging of COPD and medical treatments.
Table 1. Patient demographics and baseline characteristics.
| Demographics | Total (n=91) | ON group (n=30) | UN group (n=30) | UNWO group (n=31) | P |
|---|---|---|---|---|---|
| Male/female (n) | Aug-83 | 1/29 | 5/25 | Feb-29 | 0.165 |
| Age (years) | 71.55±8.72 | 72.30±8.8.75 | 70.534±8.47 | 71.811±9.11 | 0.724 |
| Oxygenation index (OI) | 288.08±71.99 | 299.46±65.65 | 283.94±72.17 | 280.80±78.41 | 0.565 |
| Case number of type II respiratory failure (%) | 38 (41.8) | 14 (46.7) | 13 (43.3) | 11 (35.5) | 0.663 |
| Baseline spirometry | |||||
| FVC (L) | 1.89±0.59 | 1.64±0.21 | 1.87±0.54 | 2.09±0.75 | 0.127 |
| FEV1 (L) | 0.83±0.45 | 0.63±0.15 | 0.73±0.29 | 1.01±0.59 | 0.092 |
| FEV1/predicted FEV1 (%) | 33.28±15.48 | 26.14±7.42 | 29.98±15.59 | 39.75±17.35 | 0.067 |
| FEV1/FVC (%) | 41.92±11.70 | 38.57±9.32 | 39.19±10.38 | 45.63±13.32 | 0.284 |
| PEF (L) | 2.10±1.26 | 1.74±0.46 | 1.95±0.88 | 2.41±1.71 | 0.905 |
| GOLD stages | 0.252 | ||||
| Moderate cases (%) | 5 (5.5) | 0 | 1 (3.3) | 4 (12.9) | |
| Severe cases (%) | 29 (31.9) | 8 (26.7) | 12 (40.0) | 9 (29.0) | |
| Very severe cases (%) | 57 (62.6) | 22 (73.1) | 17 (56.7) | 18 (58.1) |
ON, oxygen-driven nebulization; UN, ultrasonic nebulization; UNWO, ultrasonic nebulization with warming and oxygen; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; PEF, peak expiratory flow; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Experimental measurements for the three nebulization modalities
Table 2 shows the nebulization rates and the mouthpiece aerosol temperatures immediately before and at the end of 20-min nebulization in the three groups. Significantly higher nebulization rates were noted for the two groups using ultrasound (UN: 1.84±0.17 mL/min, UNWO: 1.84±0.17 mL/min) as compared with the ON group (0.28±0.03 mL/min) (both P<0.05). From baseline, there was a 5 °C reduction in the aerosol temperature at the end of ON, in contrast to a 1.5-2.5 °C increase at the end of two modalities of ultrasound nebulization (UN and UNWO, both P<0.01).
Table 2. Nebulization rates and aerosol temperatures in the three nebulization modalities.
| Nebulization groups | Nebulization rate (mL/min) | Temperature of mouthpiece aerosol (°C) |
|
|---|---|---|---|
| Start of nebulization | End of nebulization | ||
| ON (n=30) | 0.28±0.03 | 20.63±0.15 | 15.13±0.40 |
| UN (n=30) | 1.84±0.17* | 21.63±0.22† | 23.00±0.35*† |
| UNWO (n=31) | 1.84±0.17* | 32.81±0.78* | 35.21±0.61* |
Data are presented as mean ± SD. *, P<0.01 vs. ON group; †, P<0.01 vs. UNWO group. ON, oxygen-driven nebulization; UN, ultrasonic nebulization; UNWO, ultrasonic nebulization with warming and oxygen.
In the in vitro experiment, the mean particle size of normal saline aerosol at the end of 20-min nebulization, corresponding to the temperatures at the mouthpiece, was largest in the UN group (5.60±1.54 μm, 20 °C), followed by the ON group (5.07±0.03 μm, 20 °C) and UNWO group (4.92±0.82 μm, 35 °C). Interestingly, UNWO produced much more aerosol particles measuring 0.5-5 µm and relatively less particles >5 μm in size than UN (62.98%±10.64% vs. 39.34%±12.97%, P<0.05) (Table 3).
Table 3. Mean distribution of aerosol particle size in the three nebulization modalities.
| Size | ON (20 °C, 6 L/min) | UN (20 °C) | UNWO (32 °C, 2 L/min) |
|---|---|---|---|
| Mean size (μm) | 5.07±0.03 | 5.60±1.54*# | 4.92±0.82 |
| <0.5 μm (%) | 0 | 0 | 0 |
| 0.5-5 μm (%) | 57.69±1.84 | 39.34±12.97*# | 62.98±10.64 |
| 5-10 μm (%) | 36.21±0.37 | 40.35±0.82# | 31.65±11.14 |
| >10 μm (%) | 6.11±5.64 | 20.32±12.30*# | 5.33±4.25 |
Data are presented as mean ± SD. *, P<0.05 vs. ON group; #, P<0.05 vs. UNWO group. ON, oxygen-driven nebulization; UN, ultrasonic nebulization; UNWO, ultrasonic nebulization with warming and oxygen.
Tolerance of AECOPD patients to ON, UN, and UNWO
Classical UN was poorly tolerated to patients compared with ON or UNWO, as reflected by the least patient comfort (4.70±2.26), the fewest patients who completed the procedure [16 (53.3%)], and the shortest actual duration of nebulization (15.60±5.83 min) in this group (Table 4).
Table 4. Actual nebulization duration and patient comfort in the three groups.
| Groups | Patients who completed the 20-min nebulizing procedure (%) | Actual nebulization duration (min) | Patient comfort |
|---|---|---|---|
| ON (n=30) | 29 (96.7) | 19.87±0.73 | 6.64±1.57 |
| UN (n=30) | 16 (53.3)* | 15.60±5.83* | 4.70±2.26* |
| UNWO (n=31) | 26 (83.9)† | 18.35±3.87# | 6.40±2.26† |
*, P<0.01 vs. ON group; †, P<0.05; #, P<0.01 vs. UN group. ON, oxygen-driven nebulization; UN, ultrasonic nebulization; UNWO, ultrasonic nebulization with warming and oxygen.
As shown in Table 4, there were no significant differences in actual nebulization duration or patient comfort between the ON and UNWO groups (both P>0.05). Comparable number of patients from these two groups completed the nebulizing procedure (29 vs. 26, P>0.05).
Among the patients subjected to unscheduled termination of UN, the most frequent causes included hypoxia (n=13), wheezing (n=4) and fatigue (n=5) (Table 5).
Table 5. Causes for unscheduled termination of nebulizing procedure.
| Causes | ON group (n=1) | UN group (n=14) | UNWO group (n=5) |
|---|---|---|---|
| SpO2 of less than 85% and significantly increased shortness of breath | 0 | 13 | 0 |
| Cough and expectoration causing significantly increased shortness of breath | 0 | 2 | 3 |
| Wheezing | 0 | 4 | 1 |
| Irritating cough | 0 | 1 | 0 |
| Excessively high temperature | 0 | 0 | 2 |
| Fatigue | 1 | 5 | 1 |
SpO2, peripheral oxygen saturation; ON, oxygen-driven nebulization; UN, ultrasonic nebulization; UNWO, ultrasonic nebulization with warming and oxygen.
Sputum clearance with the three nebulization modalities
Comparisons of cough and expectoration before and after nebulization among the three groups are shown in Table 6. Apparently, induced expectoration by inhaling normal saline aerosol was more successful in patients receiving UN (n=24) or UNWO (n=29) compared with those receiving ON (n=21). Patients receiving ON experienced shorter T2E after nebulization (P<0.05), but did not show any changes in FoC (P=0.051) or 30mSV (P=0.183). In the UN and UNWO groups, there were an increase in FoC and 30mSV, and a reduction in T2E (all P<0.01) as compared with pre-nebulization. An ANCOVA was performed by using the pre-nebulization data as covariates. Compared with the ON group, patients given UN or UNWO showed shorter T2E and greater 30mSV (both P<0.01). While T2E did not differ between the UN and UNWO groups, the 30mSV appeared slightly greater in the latter, although a level of statistical significance was not reached (P=0.088).
Table 6. Comparisons of cough and expectoration before and after the start of nebulization.
| Groups | Measures | Before | After | P |
|---|---|---|---|---|
| ON (n=30) | FoC | 1.50±1.23 | 2.50±2.83 | 0.051 |
| T2E | 6.00±1.34 | 5.10±1.75 | 0.017 | |
| 30mSV (mL) | 1.20±1.99 | 1.75±1.59 | 0.183 | |
| Cases with successful expectoration (%) | 15 (50.0) | 21 (70.0) | 0.043 | |
| UN (n=30) | FoC | 1.77±1.33 | 4.03±3.63 | 0.001 |
| T2E | 6.23±1.14 | 3.63±2.08* | 0.001 | |
| 30mSV (mL) | 1.01±2.43 | 3.16±2.61* | 0.001 | |
| Cases with successful expectoration (%) | 12 (40.0) | 24 (80.0) | 0.000 | |
| UNWO (n=31) | FoC | 1.74±1.26 | 3.90±2.84 | 0.000 |
| T2E | 6.06±1.37 | 3.26±1.77** | 0.000 | |
| 30mSV (mL) | 0.85±1.07 | 4.05±2.56** | 0.000 | |
| Cases with successful expectoration (%) | 15 (48.4) | 29 (93.5)* | 0.000 |
Data are presented as mean ± SD unless otherwise specified. *, P<0.05; **, P<0.01 vs. ON group by ANCOVA. FoC, frequency of cough; T2E, time to first expectoration; 30mSV, the 30-min volume of expectorated sputum; ON, oxygen-driven nebulization; UN, ultrasonic nebulization; UNWO, ultrasonic nebulization with warming and oxygen.
Effects of the three nebulization modalities on cardiopulmonary functions
The PFT findings before and after nebulization are shown in Table 7. Before nebulization, the three groups of patients had similar profiles of pulmonary function in terms of FVC, FEV1 and PEF. After the 20-min procedure, while there was no significant change in these values in the ON group, the patients in UN group had lowered FVC (P=0.000) and FEV1 (P=0.001), and those in the UNWO group had lowered FVC (P=0.029), FEV1 (P=0.002), and PEF (P=0.000). ANCOVA with the pre-nebulization data as covariates suggested that the post-nebulization FVC and FEV1 values were significantly lower in the UN and UNWO groups than in the ON group (P<0.05), and that there was a statistically significant reduction in PEF among patients after UNWO as compared with those given UN or ON (P<0.05).
Table 7. Pulmonary function before and after nebulization.
| Group | PFT measures | Before nebulization | After nebulization | P |
|---|---|---|---|---|
| ON group (n=30) | FVC | 1.43±0.51 | 1.47±0.44 | 0.432 |
| FEV1 | 0.61±0.23 | 0.60±0.22 | 0.303 | |
| PEF | 1.65±0.59 | 1.62±0.57 | 0.571 | |
| UN group (n=30) | FVC | 1.70±0.56 | 1.50±0.53** | 0.000 |
| FEV1 | 0.75±0.28 | 0.67±0.27* | 0.001 | |
| PEF | 1.85±0.70 | 1.78±0.74 | 0.276 | |
| UNWO group (n=31) | FVC | 1.65±0.65 | 1.53±0.61 | 0.029 |
| FEV1 | 0.78±0.38 | 0.68±0.34 | 0.002 | |
| PEF | 2.18±1.15 | 1.79±0.79*† | 0.000 |
*, P< 0.05; **, P<0.01 vs. ON group by ANCOVA; †, P<0.05; Data are presented as mean ± SD unless otherwise specified. ON, oxygen-driven nebulization; UN, ultrasonic nebulization; UNWO, ultrasonic nebulization with warming and oxygen; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; PEF, peak expiratory flow.
The effects of the three nebulization modalities on SpO2, HR, RR, dyspnea, and chest tightness are shown in Table 8. Before nebulization, there were no significant differences in these clinical measures among the three groups (all P>0.05). The overall changes in these clinical measures along with all-time points (5, 10, 15 and 20 min post-nebulization) varied among the three groups:
Table 8. Physiological indicators changed during nebulization.
| Groups | Before nebulization | 5 min after nebulization | 10 min after nebulization | 15 min after nebulization | 20 min after nebulization | P |
|---|---|---|---|---|---|---|
| SpO2 | ||||||
| ON group | 92.33±3.23 | 97.47±1.48 | 97.77±1.28 | 97.63±1.30 | 97.30±1.95 | <0.001 |
| UN group | 93.57±2.25 | 92.70±2.77** | 91.33±3.14** | 90.87±3.47** | 90.70±3.16** | <0.001 |
| UNWO group | 92.42±2.91 | 95.71±2.85†† | 95.65±2.64**†† | 95.48±3.21**†† | 95.42±2.91**†† | <0.001 |
| HR | ||||||
| ON group | 90.90±11.86 | 88.40±11.75 | 87.83±11.97 | 88.43±12.26 | 87.60±11.94 | <0.001 |
| UN group | 90.67±12.44 | 92.23±12.79 | 93.47±12.57 | 93.07±12.55 | 93.30±11.80 | 0.017 |
| UNWO group | 89.35±12.78 | 87.52±11.89 | 88.42±12.02 | 88.42±12.53 | 88.87±11.45 | 0.293 |
| RR | ||||||
| ON group | 23.13±3.59 | 16.53±2.62 | 15.80±2.59 | 16.47±2.76 | 17.07±3.35 | <0.001 |
| UN group | 22.33±3.64 | 16.73±4.08 | 18.33±4.67** | 18.93±4.89* | 19.47±4.93* | <0.001 |
| UNWO group | 24.52±10.28 | 16.84±3.53 | 16.45±3.53† | 17.16±3.26 | 17.87±3.26 | <0.001 |
| Dyspnea | ||||||
| ON group | 2.23±1.16 | 1.75±0.94 | 1.55±0.86 | 1.37±0.83 | 1.30±0.81 | <0.001 |
| UN group | 2.17±1.16 | 2.12±1.15 | 2.40±1.09** | 2.53±1.07** | 2.62±1.24** | 0.009 |
| UNWO group | 2.39±1.54 | 2.11±1.35 | 2.02±1.21 | 1.97±1.10* | 1.89±1.09† | 0.049 |
| Chest tightness | ||||||
| ON group | 1.70±2.02 | 1.29±1.66 | 1.21±1.43 | 1.09±1.32 | 0.92±1.23 | 0.009 |
| UN group | 1.47±2.19 | 1.33±2.01 | 1.27±1.93 | 1.30±1.88 | 1.33±1.86 | 0.283 |
| UNWO group | 1.55±1.84 | 1.40±1.58 | 1.23±1.54 | 1.29±1.56 | 1.15±1.42 | 0.064 |
*, P<0.05; **, P<0.01 vs. ON group; †, P<0.05; ††, P<0.01 vs. UN group. SpO2, peripheral oxygen saturation; HR, heart rate; RR, respiratory rate; ON, oxygen-driven nebulization; UN, ultrasonic nebulization; UNWO, ultrasonic nebulization with warming and oxygen.
The level of SpO2 showed an increasing trend in patients given oxygen-containing nebulization (ON and UNWO groups, both P<0.001) compared with a decreasing trend in those given classical UN group (P<0.001), notwithstanding that the increase in SpO2 was smaller in the UNWO group than in the ON group;
The RR showed a decreasing trend in patients on oxygen-containing nebulization (ON and UNWO groups), compared with an increasing trend in those given UN (all P<0.001); noticeably, there was a transient reduction in RR at 5 min after start of the UN (P<0.05), but the value was increasing at the time points thereafter; measurements also indicated decreasing HR, another vital sign, in patients given ON (P<0.001), while those given UNWO did not show any change in HR (P=0.293) and those given UN experienced increasing HR over time (P<0.001);
Patients in the ON group (P<0.001) and UNWO group (P=0.049) experienced progressive improvement in dyspnea compared with a mildly worsening of the symptom in those given UN (P=0.009); while the severity of chest tightness remained unchanged throughout the procedure in patients given UN (P=0.283) or UNWO (P=0.064), those given ON (P=0.009) showed a gradual relief in the symptom.
Discussion
Nebulized inhalation is a widely-available physiotherapy that helps moisten and dilute viscous airway secretions, enhance the mucociliary motion, and may thereby facilitate airway clearance through voluntary cough. Nebulizing procedures can also have an important role in clinical treatments, because certain therapeutic agents added in the nebulizing solution may lead to direct, local actions on the airway, lowered dose of systemic medications (17), hence less treatment-related side effects (18). Owing to these benefits, nebulized inhalation has been recommended as part of therapy for patients with AECOPD in the GOLD guidelines (14). Unfortunately, the aerosol produced by currently available modalities of nebulization is either cooler than room temperature and with high oxygen flow, or lacks oxygen augmentation (as in ultrasonic nebulizer). These have been linked by studies to suboptimal efficiency of facilitated expectoration, as well as certain adverse effects [such as hypercapnia (1,2) or hypoxemia (3-5)] in specific subsets of patients with AECOPD during or after the nebulizing procedure. In the present study, classical UN was modified with additional warming and low flow-rate oxygen, so as to develop a novel modality of “ultrasound-driven, oxygen-containing” nebulized inhalation (UNWO) that may circumvent the above-mentioned disadvantages. We proceeded to validate UNWO in comparison with UN and ON regarding the sputum clearance and adverse effects in patients with AECOPD.
In present study, the normal saline rather than medication-containing solutions was chose to be nebulized to facilitate the clearance of sputum in AECOPD patients. By now, nebulized saline alone has not been explicitly recommended for sputum clearance for COPD in GOLD guidelines. But several guidelines’ recommendations might indicate this potent use. In the COPD guidelines of China (2007 and 2013 updated) (19,20) and GOLD guidelines (2009 updated and 2010 updated) (14,21), active measures including moistening airway and stimulating cough were recommended for the sputum clearance of AECOPD patients. Hypertonic saline as potent stimulus to airway narrowing has been broadly reported in sputum induction (22). Normal saline has also been reported to be potential for significant bronchoconstriction in sputum induction in adults with acute severe asthma (23) and moderate to severe COPD patients (24). But normal saline caused less bronchoconstriction than hypertonic saline, and the bronchoconstriction can be successfully reversed by bronchodilator. Furthermore, normal saline has been broadly used as diluent of various drugs in nebulized inhalation and it was reported to be effective in inducing sputum with high safety (25,26). Based on the all above, it was reasonable to choose nebulized normal saline for this pilot research to examine the safety and sputum clearance effect of the novel UNWO.
As shown by the results, expectoration was more likely to be successful in patients given UNWO (n=29) than in those given UN (n=24) or ON (n=21). In addition, UNWO was associated with a shortened T2E, increased cough frequency and volume of expectorated sputum, as compared with merely a shortened T2E after ON. These findings, which favored the use of UNWO in helping expectoration, may be in part explained by the much higher nebulization rate and the warmer aerosol measured in UNWO than in ON [(1.84±0.17) vs. (0.28±0.03) mL/min; (35.21±0.61) vs. (15.13±0.40) °C, both P<0.05].
The therapeutic effects of nebulization have been demonstrated to correlate well with the size of aerosol particles as it determines whether the aerosol can reach the peripheral bronchioles and alveoli (27). Both UN and ON can produce aerosol particles <5 µm that can enter the small airways and alveoli (1,4,28). In this study, UNWO under a working temperature of 35 °C generated more aerosol particles <5 µm (62.98%) than did UN (39.34%) and ON (57.69%) under a temperature of 20 °C, an observation consistent with the higher temperature, the lower surface tension of nebulizing solution, and hence more smaller particle size of aerosol (12). Moreover, properly warmed aerosol (near 37 °C, 100% relative humidity) is also helpful for maintaining good gas-liquid dynamics and optimal mucociliary clearance in the respiratory tract (27-29), whereas the airway mucociliary movement may be suppressed at a temperature below 30 °C (10). In the present study, the aerosol temperature was higher and closer to body temperature in UNWO (32-35 °C) before and after the 20-min nebulization than in UN (20-23 °C) and ON (15-23 °C). All these may also have contributed to the better efficiency of facilitated expectoration in the UNWO group, in terms of T2E and sputum production (30mSV).
In our clinical practices, many patients had complained of or refused classical UN because of discomfort. Some had to discontinue the procedure as a result of intolerance. Patient tolerance is therefore an important factor related to the therapeutic efficiency of a nebulization modality. Rao et al. founded that UN may aggravate airway obstruction and cause blood gas derangement in patients with obstructive lung disease (29). Similar results were also found in the present study. Compared with the reducing patient comfort, the increasing dyspnea, and shortest mean duration of nebulization, and the significantly more patients (n=14) who discontinued the procedure as noted in the UN group, UNWO was more tolerable and closer to ON. These were further reflected by the changes in SpO2 level, HR and RR throughout the nebulization. The improving SpO2 levels with oxygen-containing nebulization (ON and UNWO) and the opposite with classical UN in our study are appreciably correlated with the severity of hypoxia in patients. Paralleled with these, there was a decreasing trend in HR and RR in the ON and UNWO groups, vs. the increasing HR and RR in the UN group along all-time points. Interestingly, the reduction in RR at 5 min of UN group may be explained by patient compliance to the investigators’ instruction to take deep, slow breath early during the procedure. Nevertheless, the RR was on the rise in those patients thereafter, probably as a result of hypoventilation and increased workload of respiration. These observations suggested that UNWO may be indeed associated with milder hypoxia and better tolerance to ultrasonic nebulization in patients with AECOPD.
Over the past decades, there have been few mechanistic studies clarifying exactly how nebulized aerosol, particularly the ultrasonic mist, interfere with lung function. In general, reduced lung function after inhalation of ultrasonic aerosol was suggested to arise from more than one mechanism, including mucosal edema, bronchospasm, and temporary airway obstruction from mobilization of hydrated peripheral secretions (29,30). In the present study, the patients received a bronchodilator (400 µg salbutamol) in order to help mitigate such airway obstruction during UN or UNWO as suggested by Rao et al. (29). We speculated that the highest nebulization rate and the high percentage of aerosol particles <5 µm that can enter the small airways and alveoli with UNWO may explain the greatest decline in lung function among patients in this group. Even so, patients given UNWO felt more comfortable and less dyspneic than those given UN, possibly due to augmentation of oxygen. We also speculated that, when patient comfort and blood gas profiles are acceptable, a greater transient decline in lung function post-nebulization may suggest a better efficiency of facilitated airway clearance, such as the much greater volume of expectorated sputum after UNWO compared with post-ON. Further studies with rigorous design are needed to validate our hypothesis.
Certain limitations of this study should be acknowledged. Firstly, although this study was completely randomized and controlled, only the short-term effects of the nebulization methods were compared. Secondly, the blood gas analyses were performed by pulse oxymetry rather than arterial puncture, which would have missed other related indicators, such as SpCO2. Thirdly, none of patients had type II respiratory failure, and therefore we did not address hypercapnia as an adverse effect caused by ON. These aspects remain to be elucidated in future studies. Last but not the least, it should be noted that the scheduled 20-min of inhalation duration in present research is not unchangeable for actual use. The actual duration should be based on the tolerance of patients. To minimize the possible risk of bronchoconstriction of saline inhalation, the UNWO in AECOPD patients was under close monitoring of well-trained staffs by now. Further researches are needed to find whether UNWO could be self-service by patients at home. Pretreatment of bronchodilators is also strongly recommended.
In conclusion, UNWO may improve patients comfort and avoid hypoxia and dyspnea caused by classical UN. It may also be more effective in facilitating sputum expectoration than ON. These benefits justify the use of UNWO as ideal adjuvant physiotherapy in AECOPD patients.
Acknowledgements
The authors thank Dr. Mengzhang He and Sister Xiaohong Zeng (Ward No. 2 of Department of Respiratory Medicine, First Affiliated Hospital of Guangzhou Medical University) for their support of the present study, Dr Yi An (Pulmonary Function Unit, First Affiliated Hospital of Guangzhou Medical University) for his guidance in pulmonary function monitoring, Prof. Jiachun Lu (Faculty of Public Health, Guangzhou Medical University) for his guidance in the study design and statistical analysis, and Mr. Zhijian Tan (Department of Medical Equipment’s, First Affiliated Hospital of Guangzhou Medical University) and Vincent Medical (Dongguan) Mfg. Co. Ltd for their guidance and assistance in the ensuring safety and stability of the monitoring equipment.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: Z Zheng.
References
- 1.Boe J, Dennis JH, O’Driscoll BR, et al. European Respiratory Society Guidelines on the use of nebulizers. Eur Respir J 2001;18:228-42. [DOI] [PubMed] [Google Scholar]
- 2.Laube BL, Janssens HM, de Jongh FH, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J 2011;37:1308-31. [DOI] [PubMed] [Google Scholar]
- 3.Valderramas SR, Atallah AN. Effectiveness and safety of hypertonic saline inhalation combined with exercise training in patients with chronic obstructive pulmonary disease: a randomized trial. Respir Care 2009;54:327-33. [PubMed] [Google Scholar]
- 4.Bathoorn E, Liesker J, Postma D, et al. Safety of sputum induction during exacerbations of COPD. Chest 2007;131:432-8. [DOI] [PubMed] [Google Scholar]
- 5.Makris D, Tzanakis N, Moschandreas J, et al. Dyspnea assessment and adverse events during sputum induction in COPD. BMC Pulm Med 2006;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu AH. Effect of Oxygen Inhalation Regimen on The Oxygen Saturation of Patients with COPD Receiving Aerosol Inhalation Therapy. Journal of clinical nursing 2006;5:7-9. (in Chinese). [Google Scholar]
- 7.Williams R, Rankin N, Smith T, et al. Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit Care Med 1996;24:1920-9. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence JC. Humidification practices in the Adult Intensive Care Unit, Prince of Wales Hospital. Respir Care Clin N Am 1998;4:301-4. [PubMed] [Google Scholar]
- 9.Hasani A, Chapman TH, McCool D, et al. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis 2008;5:81-6. [DOI] [PubMed] [Google Scholar]
- 10.Liu GQ. Nursing progress on airway humidification for patients with artificial airway. Journal of clinical nursing 2009;8:64-6. (in Chinese). [Google Scholar]
- 11.Dennis JH, Stenton SC, Beach JR, et al. Jet and ultrasonic nebuliser output: use of a new method for direct measurement of aerosol output. Thorax 1990;45:728-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steckel H, Eskandar F. Factors affecting aerosol performance during nebulization with jet and ultrasonic nebulizers. Eur J Pharm Sci 2003;19:443-55. [DOI] [PubMed] [Google Scholar]
- 13.O’Callaghan C, Barry PW. The science of nebulised drug delivery. Thorax 1997;52:S31-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Global Strategy the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2009. Available online: http://www.goldcopd.org
- 15.Kendrick KR, Baxi SC, Smith RM. Usefulness of the modified 0-10 Borg scale in assessing the degree of dyspnea in patients with COPD and asthma. J Emerg Nurs 2000;26:216-22. [DOI] [PubMed] [Google Scholar]
- 16.Popov TA, Pizzichini MM, Pizzichini E, et al. Some technical factors influencing the induction of sputum for cell analysis. Eur Respir J 1995;8:559-65. [PubMed] [Google Scholar]
- 17.Gunen H, Hacievliyagil SS, Yetkin O, et al. The role of nebulised budesonide in the treatment of exacerbations of COPD. Eur Respir J 2007;29:660-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhong N. Nipping it in the bud: An inspiring mission for prevention and management of COPD. J Thorac Dis 2012;4:102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.COPD Study Group of Chinese Society of Respiratory Disease Treatment guidelines of chronic obstructive pulmonary disease (2007 updated). Chinese Journal of Tuberculosis and Respiratory Diseases 2007;30:8-17. (in Chinese). [Google Scholar]
- 20.COPD Study Group of Chinese Society of Respiratory Disease Treatment guidelines of chronic obstructive pulmonary disease (2013 updated). Chinese Journal of Tuberculosis and Respiratory Diseases 2013;36:255-64. (in Chinese). [Google Scholar]
- 21.GOLD Executive Committee. Pocket Guide to COPD Diagnosis, Management, and Prevention (updated 2010). Global Initiative for Chronic Obstructive Lung Disease. Available online: http://www.goldcopd.org/uploads/users/files/GOLD_Pocket_2010Mar31.pdf
- 22.Anderson SD. Exercise-induced asthma and the use of hypertonic saline aerosol as a bronchial challenge. Respirology 1996;1:175-81. [DOI] [PubMed] [Google Scholar]
- 23.Wark PA, Simpson JL, Hensley MJ, et al. Safety of sputum induction with isotonic saline in adults with acute severe asthma. Clin Exp Allergy 2001;31:1745-53. [DOI] [PubMed] [Google Scholar]
- 24.Taube C, Holz O, Mücke M, et al. Airway response to inhaled hypertonic saline in patients with moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1810-5. [DOI] [PubMed] [Google Scholar]
- 25.Loh LC, Eg KP, Puspanathan P, et al. A comparison of sputum induction methods: ultrasonic vs compressed-air nebulizer and hypertonic vs isotonic saline inhalation. Asian Pac J Allergy Immunol 2004;22:11-7. [PubMed] [Google Scholar]
- 26.Gao P, Gibson PG, Zhang J, et al. The safety of sputum induction in adults with acute exacerbation of COPD. Clin Respir J 2013;7:101-9. [DOI] [PubMed] [Google Scholar]
- 27.Newman SP. Aerosol deposition considerations in inhalation therapy. Chest 1985;88:152S-60S. [DOI] [PubMed] [Google Scholar]
- 28.Reychler G, Keyeux A, Cremers C, et al. Comparison of lung deposition in two types of nebulization: intrapulmonary percussive ventilation vs. jet nebulization. Chest 2004;125:502-8. [DOI] [PubMed] [Google Scholar]
- 29.Rao S, Rose ME, Rosenberg A, et al. Effects of ultrasonic nebulization on pulmonary mechanics and blood gases in obstructive pulmonary disease. Can Med Assoc J 1972;106:1081-4. [PMC free article] [PubMed] [Google Scholar]
- 30.Rodwell LT, Anderson SD. Airway responsiveness to hyperosmolar saline challenge in cystic fibrosis: a pilot study. Pediatr Pulmonol 1996;21:282-9. [DOI] [PubMed] [Google Scholar]