FIGURE 5.
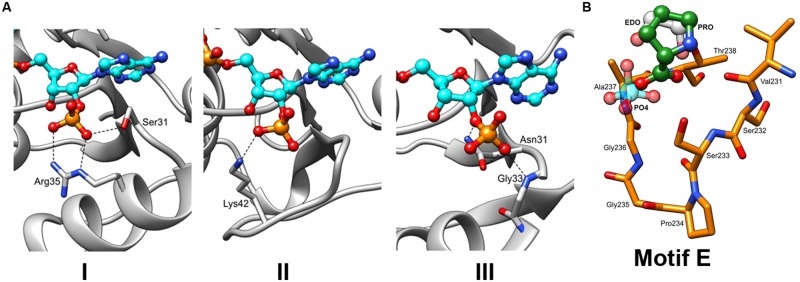
The insights into the binding of NADPH and L-Proline. (A) Three different types of interactions with the NADPH phosphate moieties can be hypothesized based on the previously characterized P5CR structures with the following residues: (Type 1) serine (S31) and arginine (R35), as observed in structure of SpP5CR (PDB id: 2AHR). (Type 2) lysine (K42) of PfP5CR (PDB id: 2RCY). (Type 3) Asparagine (N31) of NmP5CR (PDB id: 2AG8). (B) Close-up view of the motif E. Structures of enzymes with either buffer-derived molecules (phosphate ion shown in light blue and ethylene glycol molecule shown in gray) or L-proline bound (in green, as observed in the structure of SpP5CR) were superimposed. For clarity, only the structure of HsP5CR is displayed in orange.
