Abstract
Limited research has been conducted on healthcare-associated infective endocarditis (HAIE), although it is of increasing importance. The aim of this study is to compare the epidemiology, clinical characteristics, and prognosis of community-acquired IE (CA-IE) with HAIE and non-nosocomial healthcare-associated IE (NNHCA-IE). A retrospective, consecutive case-series analysis was organized and performed during the 20-year study period in Huashan Hospital, Shanghai, China. A total of 154 patients were enrolled, including 126 (81.8%) who had CA-IE and 28 (18.2%) who had HAIE, among whom 20 (71.4%) had non-nosocomial IE. Patients with HAIE compared to patients with CA-IE had poorer clinical conditions (Charlson comorbidity index ≥2: 35.7% vs. 15.1%, P = 0.012; immunosuppressive therapy: 21.4% vs. 4.0%, P = 0.005), underwent more prosthetic valve replacement (35.7% vs. 7.1%, P <0.001), had less streptococcus infection (16.7% vs. 51.1%, P = 0.007) but more atypical bacterial infection (50.0% vs. 21.1%, P = 0.017) and poorer outcomes (17.9% vs. 4.0%, P = 0.019). It is noteworthy that the results were quite similar between the comparison of patients with NNHCA-IE and those with CA-IE. Overall, in-hospital mortality was 6.5%. The IE acquisition site and low serum albumin levels (odds ratio (OR): 0.8; P = 0.04) were significantly associated with an increased risk of mortality. Nosocomial IE patients had an 8.3-fold and NNHCA-IE patients had 6.5-fold increase in the risk of mortality compared to CA-IE patients. In conclusion, HAIE and NNHCA-IE have important epidemiological and prognostic implications. Because NNHCA-IE usually occurs in patients residing in the community, it is suggested that these patients should be identified and treated by the community primary care clinical staff as early as possible.
Keywords: epidemiology, healthcare-associated infections, infective endocarditis, mortality, risk factors
Introduction
Over the past 30 years, infective endocarditis (IE) has been associated with high morbidity and mortality rates despite the improvement in health care. This apparent paradox results from changes in the disease profile, including the aging process, a shift among the predisposing factors from rheumatic heart diseases to prosthetic valve or degenerative valve disease and changes in the prevalent microbiology.1,2
Healthcare-associated IE (HAIE) is an increasingly recognized category of IE. Previous studies have shown that this classification accounted for up to 30% of IE cases in developed countries, and cases were usually acquired in hospitals.3,4 However, several studies have underlined the magnitude of the problem.5,6 Patients receiving extensive invasive medical procedures and acquiring IE in community settings are now diagnosed with non-nosocomial healthcare-associated IE (NNHCA-IE). Although medical care is becoming more and more accessible on an outpatient basis in China, research in this field is quite limited.
The aim of this study is to describe the epidemiology, clinical characteristics, and prognosis of HAIE, to compare the characteristics of community-acquired IE (CA-IE) with HAIE and NNHCA-IE and to identify the independent risk factors for in-hospital mortality.
Patients and methods
Patients and hospital setting
A retrospective, consecutive case-series analysis was organized and performed in Huashan Hospital, Shanghai, China. The data were retrieved from the hospital's standardized electronic database. All consecutive cases with IE diagnoses were selected from 1 July 1992 to 30 June 2012. The recorded variables included demographics, the presence of a predisposing cardiac condition, the Charlson comorbidity index, risk factors for transient bacteremia, risk behaviors, clinical, echocardiographic and laboratory findings, causative microorganisms, complications, treatments and outcomes. Two investigators reviewed the data independently. Patients with a first episode of diagnosed IE were included, but those with a relapse of IE were excluded, and relapse was defined as isolation of the same pathogen within a six-month period.7
Ethics statement
All data were anonymously analyzed without individual patient consent due to the retrospective nature of the study. This study protocol was approved by the institutional review boards at Huashan Hospital.
Definitions
IE diagnosis was based on the modified Duke criteria according to the clinical, echocardiographic, and microbiological findings (shown in Supplementary Table S1).8 NNHCA-IE was defined as occurring before or within 48 h of hospital admission for a patient with extensive out-of-hospital exposure to health care interventions or systems including the following: (i) receiving intravenous therapy, wound care, specialized nursing care, hemodialysis or intravenous chemotherapy within 30 days prior to the onset of IE; (ii) hospitalization for 2 or more days within 90 days before the onset of IE; (iii) residence in a nursing home or long-term care facility before hospital admission.
CA-IE was defined as patients with signs and symptoms of IE before or within 48 h of hospital admission not fulfilling the criteria for NNHCA-IE. An IE episode was classified as nosocomial when it was developed 48 h after hospital admission before the onset of signs or symptoms consistent with IE. Both NNHCA-IE and nosocomial IE were classified as HAIE.9
A predisposing cardiac condition was defined as a history of prosthetic cardiac valve replacement, congenital cardiac malformation, rheumatic and other acquired valvular dysfunction, mitral valve prolapse with valvular regurgitation or a history of an implantable pacemaker or defibrillator.10
Transthoracic echocardiogram (TTE) was performed routinely when IE was suspected. Transesophageal echocardiogram (TEE) was used to detect cases with negative TTE results after 2002. Laboratory tests were measured upon admission before initial antibiotic therapy in patients with CA-IE or NNHCA-IE or upon the onset of signs and symptoms consistent with IE in patients with nosocomial IE. These tests included hemoglobin levels, white blood cell count, platelet count, serum creatinine levels, albumin levels, and urine occult blood. Aerobic, anaerobic, and fungal blood cultures were performed routinely. Blood culture of the HACEK group (Haemophilus spp., Aggregatibacter spp, Cardiobaterium hominis, Eikenella corrodens, and Kingella kingae) and anti-bartonella, legionella, and mycoplasma antibody tests were not performed if patients had negative blood culture results. IE complications included acute left heart failure, stroke, central nervous system (CNS) infection (i.e., brain abscess and bacterial meningitis) and non-CNS embolism, which was defined as an arterial embolus causing limb ischemia or major organ infarction, including gastrointestinal, spleen, and renal infarction with obvious clinical symptoms and organ dysfunction leading to medical intervention.
Statistical analysis
The data were stored in Microsoft Access 2010 and were analyzed using Statistical Package for Social Science 20.0 (IBMcorp, Chicago, IL, USA). Categorical variables are presented as percentages and were compared using the chi-squared or Fisher's exact tests as appropriate. Continuous variables are summarized as medians and interquartile ranges, and the Mann–Whitney U test was used to evaluate the group differences. Odds ratios (OR) with 95% confidence interval (CI) were calculated using logistic regression. The variables of interest included the epidemiological data, predisposing factors, place of IE acquisition, laboratory tests, and echocardiographic findings as well as the causative pathogens. After univariate analysis, variables with P values less than 0.05 were included in forward stepwise logistic regression to identify independent variables. The site of IE acquisition was included in the regression model as coded dummy variables. P values less than 0.05 were considered statistically significant.
Results
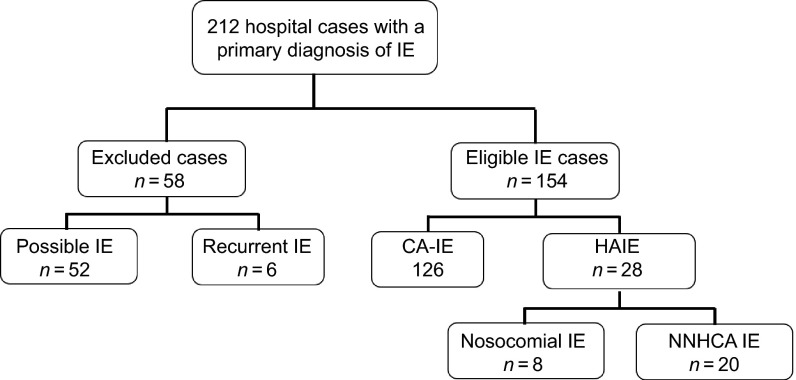
During the study period, a total of 212 cases of IE were recorded. Fifty-two cases were excluded because of a possible diagnosis and six cases of definite IE relapse were excluded (shown in Figure 1). One hundred and fifty-four definite IE cases were included. One hundred and twenty-six (81.8%) cases were CA-IE, and 28 (18.2%) cases were HAIE; 20 (71.4%) of the HAIE cases were non-nosocomial IE. Only one case was associated with intravenous drug use and was CA-IE.
Figure 1.
A flow chart of patient enrollment according to the modified Duke criteria. IE, infective endocarditis; CA, community-acquired; NNHCA, non-nosocomial healthcare-associated.
Among the patients who met the criteria for NNHCA-IE, 13 were hospitalized for more than two days within 90 days prior to admission; one received regular hemodialysis; four received wound care; and two had chemotherapy treatment within 30 days prior to the onset of IE. Six patients with nosocomial infection had undergone surgery, and the remaining two patients had a history of plasmapheresis (shown in Table 1).
Table 1. Possible source of infection in 28 cases of healthcare-associated infective endocarditis.
| NNHCA-IE | NO (%) of cases (n = 20) | Nosocomial IE | NO (%) of cases (n = 8) |
|---|---|---|---|
| Hospital admission within the previous 90 days | 13 (65.0%) | Surgery | 6 (75.0%) |
| Cardiac surgery | 5 | Cardiac surgery | 3 |
| Intestinal operation | 3 | Bone surgery | 1 |
| Pancreatic duct incision | 1 | Pneumonectomy | 1 |
| Pacemaker implantation | 1 | Reconstruction of urethral stenosis | 1 |
| Pneumonectomy | 1 | Plasmapheresis | 2 (25.0%) |
| Tonsillectomy | 1 | ||
| Retinal reattachment surgery | 1 | ||
| Chemotherapy | 2 (10.0%) | ||
| Wound care | 4 (20.0%) | ||
| Hemodialysis | 1 (5.0%) |
IE, infective endocarditis; NNHCA, non-nosocomial healthcare-associated.
Predisposing factors
The proportion of CA-IE and HAIE cases did not change throughout the study period. The median age at the time of the episodes was 46 (32–57) years, and 66.2% of patients were male. A history of prosthetic valve replacement (35.7% vs. 7.1%, P <0.001) and rheumatic heart disease (35.7% vs. 11.1%, P = 0.002) were more common in patients with HAIE than those with CA-IE. Differences were also identified between NNHCA-IE and CA-IE patients. In contrast, a history of congenital heart disease was more frequent among CA-IE patients than HAIE patients (23.8% vs. 3.6%, P = 0.016). HAIE episodes were also associated with comorbidities (Charlson comorbidity index ≥2: 35.7% vs. 15.1%, P = 0.012) and immunosuppressive therapy (21.4% vs. 4.0%, P = 0.005) (shown in Table 2). Patients with nosocomial IE were more likely to have undergone immunosuppressive therapy (50% vs. 10%, P = 0.038) (shown in Supplementary Table S2).
Table 2. Demographic characteristics, predisposing factors, clinical and echocardiographic findings, complications and outcomes of patients with infective endocarditis.
| HAIE | |||||||
|---|---|---|---|---|---|---|---|
| Total | NNHCA-IE | Nosocomial IE | |||||
| All n = 154 | CA-IE n = 126 | n = 28 | n = 20 | n = 8 | P value (CA-IE vs. HAIE) | P value (CA-IE vs. NNHCA-IE) | |
| Hospitalization between 2002 and 2012 | 118 (76.6%) | 98 (77.8%) | 20 (71.4%) | 16 (80.0%) | 4 (50.0%) | 0.473 | >0.999 |
| Median age, years (IQR) | 46 (32–57) | 47 (32–57) | 43.5 (36–56) | 44 (39–56) | 35.5 (30–53) | 0.605 | 0.955 |
| Male, n (%) | 102 (66.2%) | 84 (66.7%) | 18 (64.3%) | 13 (65.0%) | 5 (62.5%) | 0.810 | 0.884 |
| Predisposing cardiac conditions, n (%) | |||||||
| Prosthetic valve | 19 (12.3%) | 9 (7.1%) | 10 (35.7%) | 7 (35.0%) | 3 (37.5%) | <0.001 | 0.002 |
| Congenital heart disease | 31 (20.1%) | 30 (23.8%) | 1 (3.6%) | 1 (5.0%) | 0 (0.0%) | 0.016 | 0.076 |
| Rheumatic heart disease | 24 (16.9%) | 14 (11.1%) | 10 (35.7%) | 7 (35.0%) | 3 (37.5%) | 0.002 | 0.011 |
| Charlson comorbidity index≥2 | 29 (18.8%) | 19 (15.1%) | 10 (35.7%) | 7 (35.0%) | 3 (37.5%) | 0.012 | 0.053 |
| Comorbid condition, n (%) | |||||||
| Diabetes mellitus | 8 (5.2%) | 5 (4.0%) | 3 (10.7%) | 3 (15.0%) | 0 (0.0%) | 0.159 | 0.079 |
| Malignancy | 7 (4.5%) | 4 (3.2%) | 3 (10.7%) | 3 (15.0%) | 0 (0.0%) | 0.113 | 0.054 |
| Immunosuppressive therapy | 11 (7.1%) | 5 (4.0%) | 6 (21.4%) | 2 (10.0%) | 4 (50.0%) | 0.005 | 0.245 |
| Sign, n (%) | |||||||
| Fever ≥39.1°C | 80 (53.0%) | 64 (51.6%) | 16 (59.3%) | 13 (68.4%) | 3 (37.5%) | 0.471 | 0.171 |
| Heart murmur | 130 (84.4%) | 109 (86.5%) | 21 (75.0%) | 16 (80.0%) | 5 (62.5%) | 0.129 | 0.492 |
| Purpuric lesion | 40 (26.0%) | 33 (26.2%) | 7 (25.0%) | 5 (25.0%) | 2 (25.0%) | 0.897 | 0.910 |
| Echocardiographic findings, n (%) | |||||||
| Mitral valve | 85 (55.2%) | 65 (51.6%) | 20 (71.4%) | 13 (65.0%) | 7 (87.5%) | 0.056 | 0.264 |
| Aortic valve | 57 (37.0%) | 52 (41.3%) | 5 (17.9%) | 3 (15.0%) | 2 (25.0%) | 0.020 | 0.026 |
| Tricuspid valve | 10 (6.5%) | 8 (6.3%) | 2 (7.1%) | 1 (5.0%) | 1 (12.5%) | >0.999 | >0.999 |
| Vegetation | 134 (87.0%) | 110 (87.3%) | 24 (85.7%) | 16 (80.0%) | 8 (100%) | 0.051 | 0.480 |
| Surgery, n (%) | 96 (62.3%) | 80 (63.5%) | 16 (57.1%) | 12 (60.0%) | 4 (50.0%) | 0.393 | 0.764 |
| Complication, n (%) | |||||||
| Stroke | 22 (14.3%) | 16 (12.7%) | 6 (21.4%) | 4 (20.0%) | 2 (25.0%) | 0.240 | 0.480 |
| Brain abscess | 7 (4.5%) | 7 (5.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – | 0.594 |
| Acute left heart failure | 11 (7.1%) | 6 (4.8%) | 5 (17.9%) | 2 (10.0%) | 3 (37.5%) | 0.029 | 0.301 |
| Non-CNS embolism | 10 (6.5%) | 10 (7.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – | 0.358 |
| In-hospital mortality | 10 (6.5%) | 5 (4.0%) | 5 (17.9%) | 3 (15.0%) | 2 (25.0%) | 0.019 | 0.079 |
IE, infective endocarditis; CA, community-acquired; HAIE, healthcare-associated infective endocarditis; NNHCA, non-nosocomial healthcare-associated; IQR, interquartile range; CNS, central nervous system.
Clinical and echocardiographic characteristics
Fever was the most common clinical symptom. One hundred and fifty-one patients (98.1%) experienced fever. TTE was performed as a primary examination of all of the patients. Twenty-seven patients (17.5%) were diagnosed after TEE. Aortic valve involvement was more frequent in patients with CA-IE than HAIE (41.3% vs. 17.9%, P = 0.020) or NNHCA-IE (41.3% vs. 15%, P = 0.026) (shown in Table 2).
Complications and outcomes
Ninety-six (62.3%) patients underwent surgery during the hospitalization period. Fifty patients developed complications, of which stroke was the most common, accounting for 44.0% of complications. Ten (6.5%) patients died before discharge. The patients with HAIE had significantly higher in-hospital mortality than those with CA-IE (17.9% vs. 4.0%, P = 0.019) (shown in Table 2).
Microbiology
Blood cultures were taken from all patients. One hundred and eight (70.1%) cultures had positive results. Three patients sustained multiple bacterial infections. Upon comparison of the period from 1992∼2002 and from 2002∼2012, the proportion of streptococci increased from 28.0% to 50.6% (shown in Supplementary Table S3). The prevalence of other organisms did not markedly change.
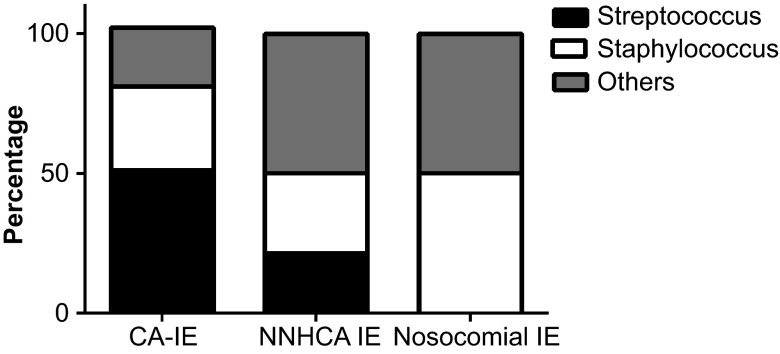
The microbiological constitutions of positive cultures differed among the three groups (shown in Figure 2). Streptococci were more common in CA-IE cases than in HAIE cases (51.1% vs. 16.7%, P = 0.007) and NNHCA-IE cases (51.1% vs. 21.4%, P = 0.038), whereaso nosocomial IE patients had streptococci. The proportions of patients with staphylococci among the CA-IE and HAIE groups were comparable (shown in Table 3). Non-streptococcus and non-staphylococcus infections were significantly higher in patients with HAIE and NNHCA-IE compared to those with community-acquired infection (50.0% vs. 21.1%, P = 0.017; 50.0% vs. 21.1%, P = 0.041). These microorganisms included gram-negative bacilli, enterococci, Candida albicans, gram-positive bacilli, Rhodococcus, Sphingomonas paucimobilis, and Aerococcus viridans. There were no differences in the microbiological spectrum found in NNHCA-IE and nosocomial IE cases (shown in Supplementary Table S4).
Figure 2.
The distribution of causative pathogens in positive cultures classified according to site of infection acquisition. IE, infective endocarditis; CA, community-acquired; NNHCA, non-nosocomial healthcare-associated
Table 3. Etiologic agents among patients with infective endocarditis.
| HAIE | |||||||
|---|---|---|---|---|---|---|---|
| Total | Nosocomial IE | NNHCA-IE | |||||
| All n = 154 | CA-IE n = 126 | n = 28 | n = 8 | n = 20 | P value (CA-IE vs. HAIE) | P value (CA-IE vs. NNHCA-IE) | |
| Culture negative, n (%) | 46 (29.9%) | 36 (28.6%) | 10 (35.7%) | 4 (50.0%) | 6 (30.0%) | 0.455 | 0.896 |
| Streptococci, n (%) | 49 (44.5%) | 46 (51.1%) | 3 (16.7%) | 0 (0.0%) | 3 (21.4%) | 0.007 | 0.038 |
| VGSa | 38 (77.6%) | 36 (78.3%) | 2 (11.1%) | 0 (0.0%) | 2 (66.7%) | 0.019 | 0.542 |
| S. bovisa | 3 (6.1%) | 3 (6.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – | – |
| Othersa | 8 (16.3%) | 7 (15.2%) | 1 (3.6%) | 0 (0.0%) | 1 (33.3%) | – | – |
| Staphylococci, n (%) | 33 (30.0%) | 27 (30.0%) | 6 (33.3%) | 2 (50.0%) | 4 (28.6%) | 0.779 | >0.999 |
| Staphylococcus aureusb | 19 (57.8%) | 16 (59.3%) | 3 (10.7%) | 0 (0.0%) | 3 (75.0%) | – | – |
| MRSAc | 3 (15.8%) | 3 (18.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – | – |
| MRCNSc | 4 (28.6%) | 3 (27.3%) | 1 (5.6%) | 1 (50.0%) | 0 (0.0%) | – | – |
| Other organismd, n (%) | 28 (25.5%) | 19 (21.1%) | 9 (50.0%) | 2 (50.0%) | 7 (50.0%) | 0.017 | 0.041 |
| Gram-negative bacilli | 8 (7.4%) | 6 (4.8%) | 2 (7.1%) | 1 (12.5%) | 1 (5.0%) | 0.638 | >0.999 |
| Enterococci | 12 (11.1%) | 9 (7.1%) | 3 (10.7%) | 1 (12.5%) | 2 (10.0%) | 0.458 | 0.628 |
| C. albicans | 2 (1.9%) | 0 (0.0%) | 2 (11.1%) | 0 (0.0%) | 2 (10.0%) | – | – |
IE, infective endocarditis; CA, community-acquired; HAIE, healthcare-associated IE; NNHCA, non-nosocomial healthcare-associated; VGS, viridans group streptococci; MRSA, methicillin-resistant S. aureus; CNS, coagulase-negative staphylococci; MRCNS, methicillin-resistant CNS.
Proportion was based on patients with streptococci.
Proportion was based on patients with staphylococci.
Proportion was based on patients with S. aureus and CNS, respectively.
Includes gram-negative bacilli, enterococci, C. albicans, gram-positive bacilli, Rhodococcus, S. paucimobilis, and A. viridans.
Risk factors for in-hospital mortality
In this study, the in-hospital mortality was 6.5%. The causes of death included stroke (3), heart failure (3), CNS infection (2), complication of surgery (1), and septic shock (1). Nosocomial infection had the highest mortality rate (25.0%, 2/8), followed by NNHCA-IE (15.0%, 3/20), whereas the mortality rate of community-acquired infection was the lowest (4.0%, 5/126). Factors contributing to in-hospital mortality included the site of acquisition of endocarditis, white blood cell counts>10 × 109/L, decreased serum albumin levels and urine occult blood ≥3+. After multivariate regression of these factors, the site of infection acquisition was significantly associated with increased mortality (P = 0.041). Compared with community-acquired infection, nosocomial infection had an 8.3-fold (95%CI: 1.0–69.0; P = 0.050) increase in mortality and NNHCA-IE had a 6.5-fold (95%CI: 1.2–36.3; P = 0.034) increase in mortality. Low albumin levels were also an independent risk factor for mortality. Each unit increase in the albumin level was associated with a 0.8-fold (95%CI: 0.7–0.9; P = 0.040) decrease in mortality (shown in Table 4).
Table 4. Risk factors for in-hospital death in patients with infective endocarditis.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Risk factors | P value | OR (95% CI) | P value | OR (95% CI) |
| Age ≥60 years old | 0.966 | 1.0 (0.2–5.1) | – | – |
| Male | 0.795 | 0.8 (0.2–3.4) | – | – |
| Smoking | 0.379 | 0.4 (0.0–3.2) | – | – |
| Community-acquired IE | 0.036 | 1 | 0.041 | – |
| Nosocomial IE | 0.026 | 8.1 (1.3–50.5) | 0.050 | 8.3 (1.0–69.0) |
| NNHCA-IE | 0.061 | 4.3 (0.9–19.5) | 0.034 | 6.5 (1.2–36.3) |
| Charlson comorbidity index ≥2 | 0.922 | 1.1 (0.2–5.4) | – | – |
| Predisposing cardiac conditions | 0.785 | 0.8 (0.2–3.1) | – | – |
| Prosthetic valve | 0.817 | 0.8 (0.1–6.5) | – | – |
| Congenital heart disease | 0.422 | 0.4 (0.1–3.5) | – | – |
| WBC> 10 × 109/L | 0.035 | 4.1 (1.1–15.5) | – | – |
| Serum creatinine levels>110 (μmol/L) | 0.225 | 2.8 (0.5–15.0) | – | – |
| Albumin levels (g/L) | 0.003 | 0.8 (0.7–0.9) | 0.04 | 0.8 (0.7–0.9) |
| Urine occult blood ≥3+ | 0.017 | 5.0 (1.3–19.0) | – | – |
| Mitral valve | 0.123 | 3.5 (0.7–17.0) | – | – |
| Aortic valve | 0.263 | 0.4 (0.1–2.0) | – | – |
| Vegetation | 0.500 | 0.6 (0.1–2.9) | – | – |
| Negative blood culture | 0.473 | 1.6 (0.4–6.0) | – | – |
| Streptococcus infectiona | 0.180 | 0.2 (0.0–2.0) | – | – |
| Staphylococcus infectiona | 0.130 | 1.9 (0.8–4.5) | – | – |
OR, odds ratio; CI, confidence interval; IE, infective endocarditis; NNHCA, non-nosocomial healthcare-associated; WBC, white blood cell.
Analysis was based on 108 positive cultures.
Discussion
This study described the epidemiology and prognosis of HAIE (including cases of NNHCA-IE) in Huashan Hospital, Shanghai, China. Using this broader definition, we found that HAIE and NNHCA-IE represented almost 20% and 13% of all episodes of IE, respectively, which is similar to the data derived from developed areas (21.5%–34.0% and 5.5%–15.7%, respectively).3,5,11 Among HAIE cases, up to 71.4% were non-nosocomial IE. In studies from Spain and Taiwan, NNHCA-IE only accounted for 19.3% and 57.7% of HAIE cases, respectively.3,11 This finding reflects the current trends in epidemiology in China, where the length of hospitalization after surgery is shortened and outpatient medical interventions have increased. These changes have been followed by an increased risk of healthcare-associated bacteremia resulting in NNHCA-IE. NNHCA-IE requires more attention, as it is usually primarily identified as a community-acquired infection, resulting in a delay of optimal treatment.
HAIE tended to affect patients with poor clinical conditions. Additionally, patients with prosthetic valve replacement and rheumatic heart disease were more likely to have HAIE. These comorbidities were linked to close contact with the health-care system and a subsequent increased risk of acquiring bacteremia and IE. The majority of cases with congenital heart disease were diagnosed only after the development of IE, implying that these patients had similar medical exposures to the patients with community-acquired infections.
Based on a review of the literature published in developed countries, the presence of a positive blood culture varied from 83% to 96%.10,12,13,14 Compared with other studies, the positive culture rate of this study was low. However, the positive culture rate was approximately 38.5%–70.1% in China,15,16,17,18 which is similar to the data published from India.19,20 This finding could be related to the use of antibiotics. Most patients in Huashan Hospital had been referred. They usually had a history of antibiotic use prior to the microbiological exam. In Huashan Hospital, the blood cultures of patients in the HACEK group, serology tests for bartonella, legionella, and mycoplasma as well as broad range polymerase chain reaction were not performed. These factors resulted in low microbiology detection. It has been suggested that patients with suspected bloodstream infection should receive blood cultures to rule out or confirm the diagnosis because a positive result is of great clinical significance.
Although several studies have suggested that staphylococci are the most common pathogens,2,11,21 streptococci still predominate in this setting, especially in CA-IE cases. This predominance could be related to the younger age, the presence of underlying congenital heart disease, and the rates of community acquisition observed among the patients in this study. The causative pathogens of CA-IE and HAIE cases were quite different. There was a higher proportion of non-streptococcus and non-staphylococcus infection in patients with HAIE, as previously mentioned by other authors.3,6,22 The spectrum of pathogens found in NNHCA-IE cases had a similar pattern to those found in HAIE cases. These organisms often have a healthcare-associated origin, are caused by virulent and resistant organisms and result in high rates of complications and high mortality rates.23,24,25 These findings emphasized the importance of the site of acquisition when evaluating the causes of IE and initiating treatment with antibiotics alone or in combination with surgery.
Two risk factors for in-hospital mortality were identified, including the site of IE acquisition and low serum albumin levels, which are consistent with previous reports that suggested a higher mortality rate in patients with healthcare-associated infections.3,5,6,11,26 This finding could be the result of composite factors, including the frequency of comorbid conditions, non-typical microorganism infection, and increased involvement of the prosthetic valve.21,27 Additionally, nosocomial IE frequently affects the mitral and aortic valves. Although these factors were not found to be related to the disease prognosis, their association with poor outcomes has been described in previous studies.28,29 Low albumin levels were linked with mortality.30 The mechanism is not yet clear. However, albumin levels may merely be an integrative barometer of multiple adverse events, including heart failure,31 a hypercoagulable state,32 malnutrition and cachexia, and renal impairment. These events have been confirmed to be risk factors for mortality in IE patients.10,13,33,34,35 Monitoring serum albumin levels may provide important prognostic information and may prompt attempts to correct worsening inflammation, nutrition, congestion, or hepatic and renal dysfunction.
This study has several limitations. It was performed in a tertiary medical center where most patients were transferred for further treatment and were difficult to diagnose, resulting in long-term disease and negative blood culture results. Therefore, these results should not be generalized to other patient groups. In addition, the long-term prognosis of the disease cannot be evaluated, as these patients were not followed up longitudinally. To maintain the large sample size in a single medical center, the study period took place over a long period of time. Changes in microbiology trends and treatment regimens could affect the observed patient prognoses. Therefore, multiple-center prospective cohort studies are recommended.
In conclusion, this study reveals that HAIE is an important health problem and that NNHCA-IE is prevalent in this patient population. The infection usually affects patients in poor clinical condition with prosthetic valve replacement or rheumatic heart disease. HAIE is mainly caused by staphylococci and atypical bacterial infection and is associated with considerable in-hospital mortality. Therefore, it is important that clinicians closely inquire about the patients' medical history exposures, especially when discriminating NNHCA-IE from CA-IE episodes, and consequently adjust treatment to improve patients' outcomes.
Footnotes
Supplementary information for this article can be found on the Emerging Microbes & Infections' website (http://www.nature.com/EMI).
Supplementary Information
References
- Que YA, Moreillon P. Infective endocarditis. Nat Rev Cardiol. 2011;8:322–336. doi: 10.1038/nrcardio.2011.43. [DOI] [PubMed] [Google Scholar]
- Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU. Infective endocarditis in the U.S., 1998-2009: a nationwide study. PLoS One. 2013;8:e60033. doi: 10.1371/journal.pone.0060033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Hidalgo N, Almirante B, Tornos P, et al. Contemporary epidemiology and prognosis of healthcare-associated infective endocarditis. Clin Infect Dis. 2008;47:1287–1297. doi: 10.1086/592576. [DOI] [PubMed] [Google Scholar]
- Martin-Davila P, Fortun J, Navas E, et al. Nosocomial endocarditis in a tertiary hospital: an increasing trend in native valve cases. Chest. 2005;128:772–779. doi: 10.1378/chest.128.2.772. [DOI] [PubMed] [Google Scholar]
- Giannitsioti E, Skiadas I, Antoniadou A, et al. Nosocomial vs.. community-acquired infective endocarditis in Greece: changing epidemiological profile and mortality risk. Clin Microbiol Infect. 2007;13:763–769. doi: 10.1111/j.1469-0691.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- Benito N, Miro JM, de Lazzari E, et al. Health care-associated native valve endocarditis: importance of non-nosocomial acquisition. Ann Intern Med. 2009;150:586–594. doi: 10.7326/0003-4819-150-9-200905050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu VH, Sexton DJ, Cabell CH, et al. Repeat infective endocarditis: differentiating relapse from reinfection. Clin Infect Dis. 2005;41:406–409. doi: 10.1086/431590. [DOI] [PubMed] [Google Scholar]
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- Sy RW, Chawantanpipat C, Richmond DR, Kritharides L. Development and validation of a time-dependent risk model for predicting mortality in infective endocarditis. Eur Heart J. 2011;32:2016–2026. doi: 10.1093/eurheartj/ehp085. [DOI] [PubMed] [Google Scholar]
- Wu KS, Lee SS, Tsai HC, et al. Non-nosocomial healthcare-associated infective endocarditis in Taiwan: an underrecognized disease with poor outcome. BMC Infect Dis. 2011;11:221. doi: 10.1186/1471-2334-11-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalani T, Cabell CH, Benjamin DK, et al. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation. 2010;121:1005–1013. doi: 10.1161/CIRCULATIONAHA.109.864488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga M, Niwa K, Niwa A, et al. Risk factors for in-hospital mortality during infective endocarditis in patients with congenital heart disease. Am J Cardiol. 2008;101:114–118. doi: 10.1016/j.amjcard.2007.07.054. [DOI] [PubMed] [Google Scholar]
- Fortun J, Centella T, Martin-Davila P, et al. Infective endocarditis in congenital heart disease: a frequent community-acquired complication. Infection. 2013;41:167–174. doi: 10.1007/s15010-012-0326-6. [DOI] [PubMed] [Google Scholar]
- Li L, Wang H, Wang L, Pu J, Zhao H. Changing profile of infective endocarditis: a clinicopathologic study of 220 patients in a single medical center from 1998 through 2009. Tex Heart Inst J. 2014;41:491–498. doi: 10.14503/THIJ-13-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XZ, Li XY, Que CL, Lv Y. Underlying heart disease and microbiological spectrum of adult infective endocarditis in one Chinese university hospital: a 10-year retrospective study. Intern Med J. 2013;43:1303–1309. doi: 10.1111/imj.12248. [DOI] [PubMed] [Google Scholar]
- Xie H, Hu B, Zhou C, Zhou Q, Gao X.[An analysis of clinical characteristics, etiologies and prognosis of 218 patients with infective endocarditis] Zhonghua Nei Ke Za Zhi 201453363–367.Chinese. [PubMed] [Google Scholar]
- Wang P, Lu J, Wang H, et al. [Clinical characteristics of infective endocarditis: analysis of 368 cases] Zhonghua Xin Xue Guan Bing Za Zhi 201442140–144.Chinese. [PubMed] [Google Scholar]
- Math RS, Sharma G, Kothari SS, et al. Prospective study of infective endocarditis from a developing country. Am Heart J. 2011;162:633–638. doi: 10.1016/j.ahj.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Gupta A, Gupta A, Kaul U, Varma A. Infective endocarditis in an Indian setup: are we entering the ‘modern' era? Indian J Crit Care Med. 2013;17:140–147. doi: 10.4103/0972-5229.117041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athan E, Chu VH, Tattevin P, et al. Clinical characteristics and outcome of infective endocarditis involving implantable cardiac devices. JAMA. 2012;307:1727–1735. doi: 10.1001/jama.2012.497. [DOI] [PubMed] [Google Scholar]
- Sy RW, Kritharides L. Health care exposure and age in infective endocarditis: results of a contemporary population-based profile of 1536 patients in Australia. Eur Heart J. 2010;31:1890–1897. doi: 10.1093/eurheartj/ehq110. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Murdoch DR, Sexton DJ, et al. Risk factors for infective endocarditis in patients with enterococcal bacteremia: a case-control study. Infection. 2004;32:72–77. doi: 10.1007/s15010-004-2036-1. [DOI] [PubMed] [Google Scholar]
- McDonald JR, Olaison L, Anderson DJ, et al. Enterococcal endocarditis: 107 cases from the international collaboration on endocarditis merged database. Am J Med. 2005;118:759–766. doi: 10.1016/j.amjmed.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Durante-Mangoni E, Tripodi MF, Albisinni R, Utili R. Management of gram-negative and fungal endocarditis. Int J Antimicrob Agents. 2010;36 Suppl 2:S40–S45. doi: 10.1016/j.ijantimicag.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Gilleece A, Fenelon L. Nosocomial infective endocarditis. J Hosp Infect. 2000;46:83–88. doi: 10.1053/jhin.2000.0802. [DOI] [PubMed] [Google Scholar]
- Mirabel M, Sonneville R, Hajage D, et al. Long-term outcomes and cardiac surgery in critically ill patients with infective endocarditis. Eur Heart J. 2014;35:1195–1204. doi: 10.1093/eurheartj/eht303. [DOI] [PubMed] [Google Scholar]
- Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheugt CL, Uiterwaal CS, van der Velde ET, et al. Turning 18 with congenital heart disease: prediction of infective endocarditis based on a large population. Eur Heart J. 2011;32:1926–1934. doi: 10.1093/eurheartj/ehq485. [DOI] [PubMed] [Google Scholar]
- Roca B, Marco JM. Presentation and outcome of infective endocarditis in Spain: a retrospective study. Int J Infect Dis. 2007;11:198–203. doi: 10.1016/j.ijid.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Jabbour R, Ling HZ, Norrington K, et al. Serum albumin changes and multivariate dynamic risk modelling in chronic heart failure. Int J Cardiol. 2014;176:437–443. doi: 10.1016/j.ijcard.2014.07.096. [DOI] [PubMed] [Google Scholar]
- Bakal RB, Karakoyun S, Kahveci G, et al. [Relationship between D-dimer and systemic embolism in patients with infective endocarditis] Turk Kardiyol Dern Ars. 2013;41:589–594.. doi: 10.5543/tkda.2013.02058. [DOI] [PubMed] [Google Scholar]
- Revilla A, Lopez J, Vilacosta I, et al. Clinical and prognostic profile of patients with infective endocarditis who need urgent surgery. Eur Heart J. 2007;28:65–71. doi: 10.1093/eurheartj/ehl315. [DOI] [PubMed] [Google Scholar]
- Lopez J, Revilla A, Vilacosta I, et al. Age-dependent profile of left-sided infective endocarditis: a 3-center experience. Circulation. 2010;121:892–897. doi: 10.1161/CIRCULATIONAHA.109.877365. [DOI] [PubMed] [Google Scholar]
- Regueiro A, Falces C, Cervera C, et al. Risk factors for pericardial effusion in native valve infective endocarditis and its influence on outcome. Am J Cardiol. 2013;112:1646–1651. doi: 10.1016/j.amjcard.2013.07.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.