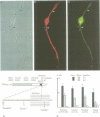
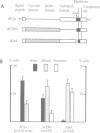
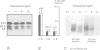Abstract
We have analysed the axonal sorting signals of amyloid precursor protein (APP). Wild-type and mutant versions of human APP were expressed in hippocampal neurons using the Semliki forest virus system. We show that wild-type APP and mutations implicated in Alzheimer's disease and another brain beta-amyloidosis are sorted to the axon. By analysis of deletion mutants we found that the membrane-inserted APP ectodomain but not the cytoplasmic tail is required for axonal sorting. Systematic deletions of the APP ectodomain identified two regions required for axonal delivery: one encoded by exons 11-15 in the carbohydrate domain, the other encoded by exons 16-17 in the juxtamembraneous beta-amyloid domain. Treatment of the cells with the N-glycosylation inhibitor tunicamycin induced missorting of wild-type APP, supporting the importance of glycosylation in axonal sorting of APP. The data revealed a hierarchy of sorting signals on APP: the beta-amyloid-dependent membrane proximal signal was the major contributor to axonal sorting, while N-glycosylation had a weaker effect. Furthermore, recessive somatodendritic signals, most likely in the cytoplasmic tail, directed the protein to the dendrites when the ectodomain was deleted. Analysis of detergent solubility of APP and another axonally delivered protein, hemagglutinin, demonstrated that only hemagglutinin formed CHAPS-insoluble complexes, suggesting distinct mechanisms of axonal sorting for these two proteins. This study is the first delineation of sorting requirements of an axonally targeted protein in polarized neurons and indicates that the beta-amyloid domain plays a major role in axonal delivery of APP.
Full text
PDF

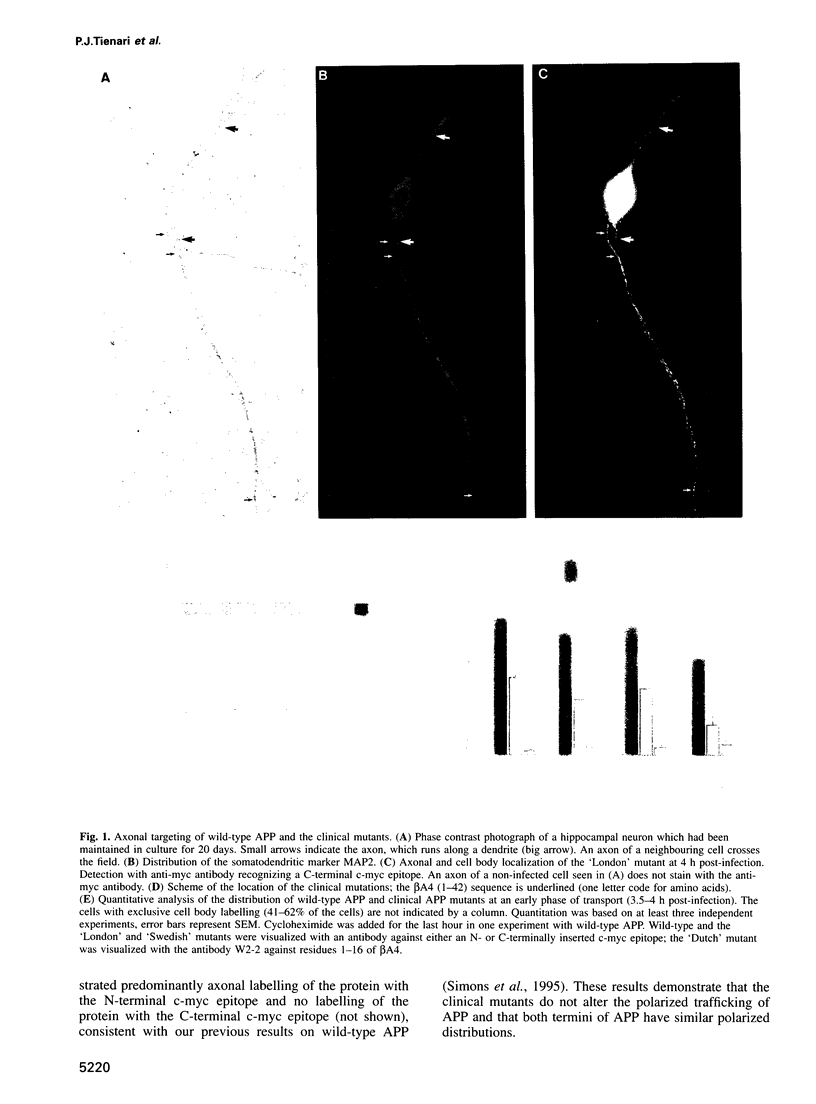








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allinquant B., Moya K. L., Bouillot C., Prochiantz A. Amyloid precursor protein in cortical neurons: coexistence of two pools differentially distributed in axons and dendrites and association with cytoskeleton. J Neurosci. 1994 Nov;14(11 Pt 2):6842–6854. doi: 10.1523/JNEUROSCI.14-11-06842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroeti B., Mostov K. E. Polarized sorting of the polymeric immunoglobulin receptor in the exocytotic and endocytotic pathways is controlled by the same amino acids. EMBO J. 1994 May 15;13(10):2297–2304. doi: 10.1002/j.1460-2075.1994.tb06513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso G. L., Takei K., Gandy S. E., Matteoli M., Mundigl O., Greengard P., De Camilli P. Morphologic and biochemical analysis of the intracellular trafficking of the Alzheimer beta/A4 amyloid precursor protein. J Neurosci. 1994 May;14(5 Pt 2):3122–3138. doi: 10.1523/JNEUROSCI.14-05-03122.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M., Teplow D. B., Selkoe D. J. Generation of amyloid beta protein from its precursor is sequence specific. Neuron. 1995 Mar;14(3):661–670. doi: 10.1016/0896-6273(95)90323-2. [DOI] [PubMed] [Google Scholar]
- Craig A. M., Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Craessaerts K., Dewachter I., Moechars D., Greenberg B., Van Leuven F., Van den Berghe H. Basolateral secretion of amyloid precursor protein in Madin-Darby canine kidney cells is disturbed by alterations of intracellular pH and by introducing a mutation associated with familial Alzheimer's disease. J Biol Chem. 1995 Feb 24;270(8):4058–4065. doi: 10.1074/jbc.270.8.4058. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Craessaerts K., Van Leuven F., Van Den Berghe H. Exchanging the extracellular domain of amyloid precursor protein for horseradish peroxidase does not interfere with alpha-secretase cleavage of the beta-amyloid region, but randomizes secretion in Madin-Darby canine kidney cells. J Biol Chem. 1995 Dec 22;270(51):30310–30314. doi: 10.1074/jbc.270.51.30310. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Simons M., Multhaup G., Van Leuven F., Beyreuther K., Dotti C. G. Production of intracellular amyloid-containing fragments in hippocampal neurons expressing human amyloid precursor protein and protection against amyloidogenesis by subtle amino acid substitutions in the rodent sequence. EMBO J. 1995 Oct 16;14(20):4932–4938. doi: 10.1002/j.1460-2075.1995.tb00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W. P., Nickoloff J. A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992 Jan;200(1):81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Kartenbeck J., Simons K. Polarized distribution of the viral glycoproteins of vesicular stomatitis, fowl plague and Semliki Forest viruses in hippocampal neurons in culture: a light and electron microscopy study. Brain Res. 1993 Apr 30;610(1):141–147. doi: 10.1016/0006-8993(93)91227-j. [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Parton R. G., Simons K. Polarized sorting of glypiated proteins in hippocampal neurons. Nature. 1991 Jan 10;349(6305):158–161. doi: 10.1038/349158a0. [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990 Jul 13;62(1):63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- Dyrks T., Dyrks E., Mönning U., Urmoneit B., Turner J., Beyreuther K. Generation of beta A4 from the amyloid protein precursor and fragments thereof. FEBS Lett. 1993 Nov 29;335(1):89–93. doi: 10.1016/0014-5793(93)80446-2. [DOI] [PubMed] [Google Scholar]
- Dyrks T., Weidemann A., Multhaup G., Salbaum J. M., Lemaire H. G., Kang J., Müller-Hill B., Masters C. L., Beyreuther K. Identification, transmembrane orientation and biogenesis of the amyloid A4 precursor of Alzheimer's disease. EMBO J. 1988 Apr;7(4):949–957. doi: 10.1002/j.1460-2075.1988.tb02900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A., Caceres A., Kosik K. S. Intraneuronal compartments of the amyloid precursor protein. J Neurosci. 1993 Jul;13(7):3112–3123. doi: 10.1523/JNEUROSCI.13-07-03112.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K., Kobayashi T., Kurzchalia T. V., Simons K. Glycosphingolipid-enriched, detergent-insoluble complexes in protein sorting in epithelial cells. Biochemistry. 1993 Jun 29;32(25):6365–6373. doi: 10.1021/bi00076a009. [DOI] [PubMed] [Google Scholar]
- Fiedler K., Simons K. The role of N-glycans in the secretory pathway. Cell. 1995 May 5;81(3):309–312. doi: 10.1016/0092-8674(95)90380-1. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goate A., Chartier-Harlin M. C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991 Feb 21;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Haass C., Koo E. H., Capell A., Teplow D. B., Selkoe D. J. Polarized sorting of beta-amyloid precursor protein and its proteolytic products in MDCK cells is regulated by two independent signals. J Cell Biol. 1995 Feb;128(4):537–547. doi: 10.1083/jcb.128.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Selkoe D. J. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993 Dec 17;75(6):1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- Hansson H. A., Holmgren J., Svennerholm L. Ultrastructural localization of cell membrane GM1 ganglioside by cholera toxin. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3782–3786. doi: 10.1073/pnas.74.9.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E., Parton R. G., Hunziker W., Simons K., Dotti C. G. Transcytosis of the polymeric immunoglobulin receptor in cultured hippocampal neurons. Curr Biol. 1993 Oct 1;3(10):635–644. doi: 10.1016/0960-9822(93)90061-r. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Koo E. H., Sisodia S. S., Archer D. R., Martin L. J., Weidemann A., Beyreuther K., Fischer P., Masters C. L., Price D. L. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia T. V., Dupree P., Parton R. G., Kellner R., Virta H., Lehnert M., Simons K. VIP21, a 21-kD membrane protein is an integral component of trans-Golgi-network-derived transport vesicles. J Cell Biol. 1992 Sep;118(5):1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E., Carman M. D., Fernandez-Madrid I. J., Power M. D., Lieberburg I., van Duinen S. G., Bots G. T., Luyendijk W., Frangione B. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990 Jun 1;248(4959):1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- Liljeström P., Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (N Y) 1991 Dec;9(12):1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin K. S., Simons K. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell. 1983 Aug;34(1):233–243. doi: 10.1016/0092-8674(83)90154-x. [DOI] [PubMed] [Google Scholar]
- Matter K., Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994 Aug;6(4):545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Matter K., Whitney J. A., Yamamoto E. M., Mellman I. Common signals control low density lipoprotein receptor sorting in endosomes and the Golgi complex of MDCK cells. Cell. 1993 Sep 24;74(6):1053–1064. doi: 10.1016/0092-8674(93)90727-8. [DOI] [PubMed] [Google Scholar]
- Mullan M., Crawford F., Axelman K., Houlden H., Lilius L., Winblad B., Lannfelt L. A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992 Aug;1(5):345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- Olkkonen V. M., Liljeström P., Garoff H., Simons K., Dotti C. G. Expression of heterologous proteins in cultured rat hippocampal neurons using the Semliki Forest virus vector. J Neurosci Res. 1993 Jul 1;35(4):445–451. doi: 10.1002/jnr.490350412. [DOI] [PubMed] [Google Scholar]
- Parton R. G. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J Histochem Cytochem. 1994 Feb;42(2):155–166. doi: 10.1177/42.2.8288861. [DOI] [PubMed] [Google Scholar]
- Pietrini G., Suh Y. J., Edelmann L., Rudnick G., Caplan M. J. The axonal gamma-aminobutyric acid transporter GAT-1 is sorted to the apical membranes of polarized epithelial cells. J Biol Chem. 1994 Feb 11;269(6):4668–4674. [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Powell S. K. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- Sandbrink R., Masters C. L., Beyreuther K. Beta A4-amyloid protein precursor mRNA isoforms without exon 15 are ubiquitously expressed in rat tissues including brain, but not in neurons. J Biol Chem. 1994 Jan 14;269(2):1510–1517. [PubMed] [Google Scholar]
- Scheiffele P., Peränen J., Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995 Nov 2;378(6552):96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- Schubert W., Prior R., Weidemann A., Dircksen H., Multhaup G., Masters C. L., Beyreuther K. Localization of Alzheimer beta A4 amyloid precursor protein at central and peripheral synaptic sites. Brain Res. 1991 Nov 1;563(1-2):184–194. doi: 10.1016/0006-8993(91)91532-6. [DOI] [PubMed] [Google Scholar]
- Simons K., Zerial M. Rab proteins and the road maps for intracellular transport. Neuron. 1993 Nov;11(5):789–799. doi: 10.1016/0896-6273(93)90109-5. [DOI] [PubMed] [Google Scholar]
- Simons M., Ikonen E., Tienari P. J., Cid-Arregui A., Mönning U., Beyreuther K., Dotti C. G. Intracellular routing of human amyloid protein precursor: axonal delivery followed by transport to the dendrites. J Neurosci Res. 1995 May 1;41(1):121–128. doi: 10.1002/jnr.490410114. [DOI] [PubMed] [Google Scholar]
- Simons M., de Strooper B., Multhaup G., Tienari P. J., Dotti C. G., Beyreuther K. Amyloidogenic processing of the human amyloid precursor protein in primary cultures of rat hippocampal neurons. J Neurosci. 1996 Feb 1;16(3):899–908. doi: 10.1523/JNEUROSCI.16-03-00899.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia S. S. Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6075–6079. doi: 10.1073/pnas.89.13.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger-Ness A., Bennett M. K., Antony C., Simons K. Distinct transport vesicles mediate the delivery of plasma membrane proteins to the apical and basolateral domains of MDCK cells. J Cell Biol. 1990 Sep;111(3):987–1000. doi: 10.1083/jcb.111.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Wertkin A. M., Turner R. S., Pleasure S. J., Golde T. E., Younkin S. G., Trojanowski J. Q., Lee V. M. Human neurons derived from a teratocarcinoma cell line express solely the 695-amino acid amyloid precursor protein and produce intracellular beta-amyloid or A4 peptides. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9513–9517. doi: 10.1073/pnas.90.20.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T., Selkoe D. J., Koo E. H. Trafficking of cell surface beta-amyloid precursor protein: retrograde and transcytotic transport in cultured neurons. J Cell Biol. 1995 Apr;129(2):431–442. doi: 10.1083/jcb.129.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa K., Odaka A., Suzuki N., Ihara Y. GM1 ganglioside-bound amyloid beta-protein (A beta): a possible form of preamyloid in Alzheimer's disease. Nat Med. 1995 Oct;1(10):1062–1066. doi: 10.1038/nm1095-1062. [DOI] [PubMed] [Google Scholar]
- Ying Y. S., Anderson R. G., Rothberg K. G. Each caveola contains multiple glycosyl-phosphatidylinositol-anchored membrane proteins. Cold Spring Harb Symp Quant Biol. 1992;57:593–604. doi: 10.1101/sqb.1992.057.01.065. [DOI] [PubMed] [Google Scholar]
- de Hoop M., von Poser C., Lange C., Ikonen E., Hunziker W., Dotti C. G. Intracellular routing of wild-type and mutated polymeric immunoglobulin receptor in hippocampal neurons in culture. J Cell Biol. 1995 Sep;130(6):1447–1459. doi: 10.1083/jcb.130.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]