Figure 2.
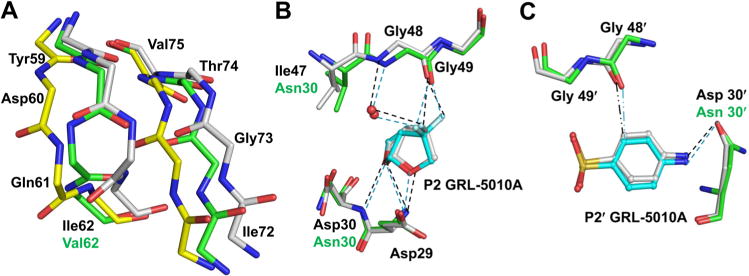
Polar interactions of PR20 with GRL-5010A. (A) The residues 59–64 at the base of the flaps were observed in two alternative conformations with the peptides of Asp 60 and Gln 61 flipped in one conformation. The corresponding residues in the adjacent strand comprising Val 71–Thr 74 also show two alternative conformations. The two conformations of the PR20 main chain atoms are colored by element type with green and yellow carbons, respectively, while the wild-type PR main chain atoms are shown with gray carbons. The side chains of PR20 have been removed for the sake of clarity. (B) The P2 gem-difluorines of GRL-5010A form strong interactions with the carbonyl of Gly 48 in the flaps of PR20 in addition to the water meditated hydrogen bond with the amide of Gly 48. In this and subsequent figures, PR20 and wild-type PR residues are colored by element type with green and white carbons, respectively. GRL-5010A is shown in ball and stick representation with cyan carbons. (C) The P2′ aniline group of GRL-5010A forms a C–H⋯O interaction (··–) with the carbonyl oxygen of Gly 48 in the flap. The P2′group of GRL-5010A also forms a hydrogen bond with the Asn 30 side chain of the active site mutation D30N in PR20.
