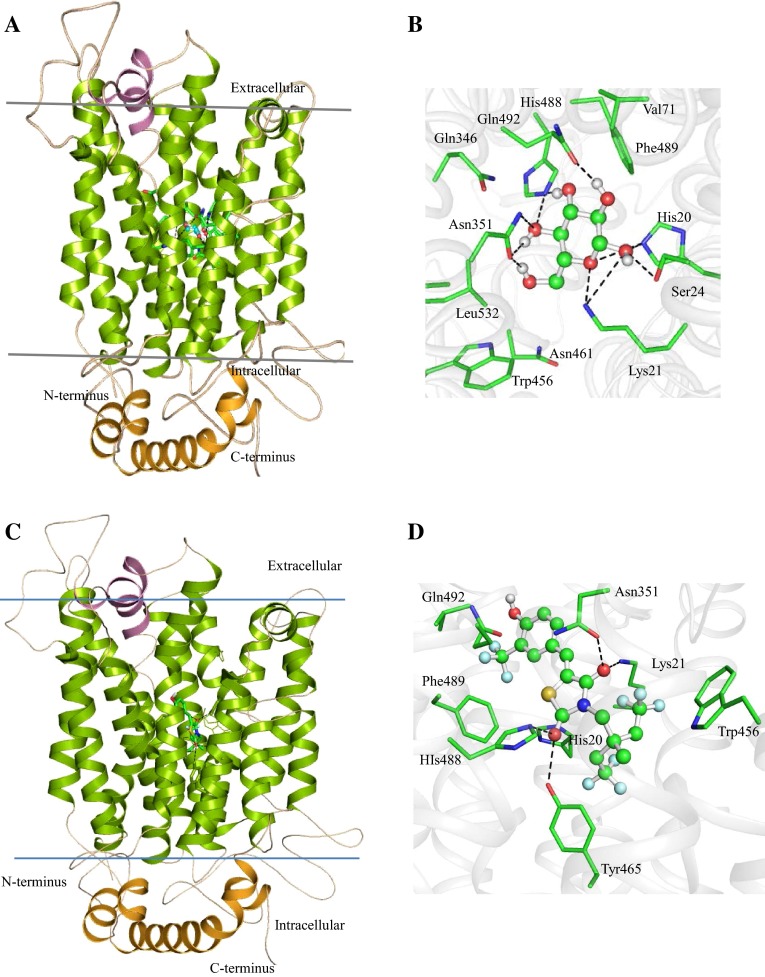
Fig. 1.
The structure of HP B9WFH2 bounded to BGC. a The overall structure of HP B9WFH2 comprised 15 transmembrane helices (green), 2 small extracellular helices (pink) and 4 intracellular helices interconnected (orange) with loops. b BGC docked in the HP B9WFH2. The active site residues are shown in stick and BGC in ball and stick model. The hydroxyl groups of BGC are involved in polar interaction (black dotted lines) with residues Lys21 and His20, Aromatic residues Phe489 and Trp456 present in the vicinity of BGC may involve in regulation glucose transport. c Showing GLUT inhibitor (5-(4-hydroxy-3-trifluoromethyl benzylidene)-3-[4,4,4-trifluoro-2-methyl-2-(2,2,2-trifluoroethyl) butyl] thiazolidine-2,4-dione) binds HP B9WFH2 at the same region where BGC binds to the HP B9WFH2. d Residues interacts with the GLUT inhibitors are shown in stick model