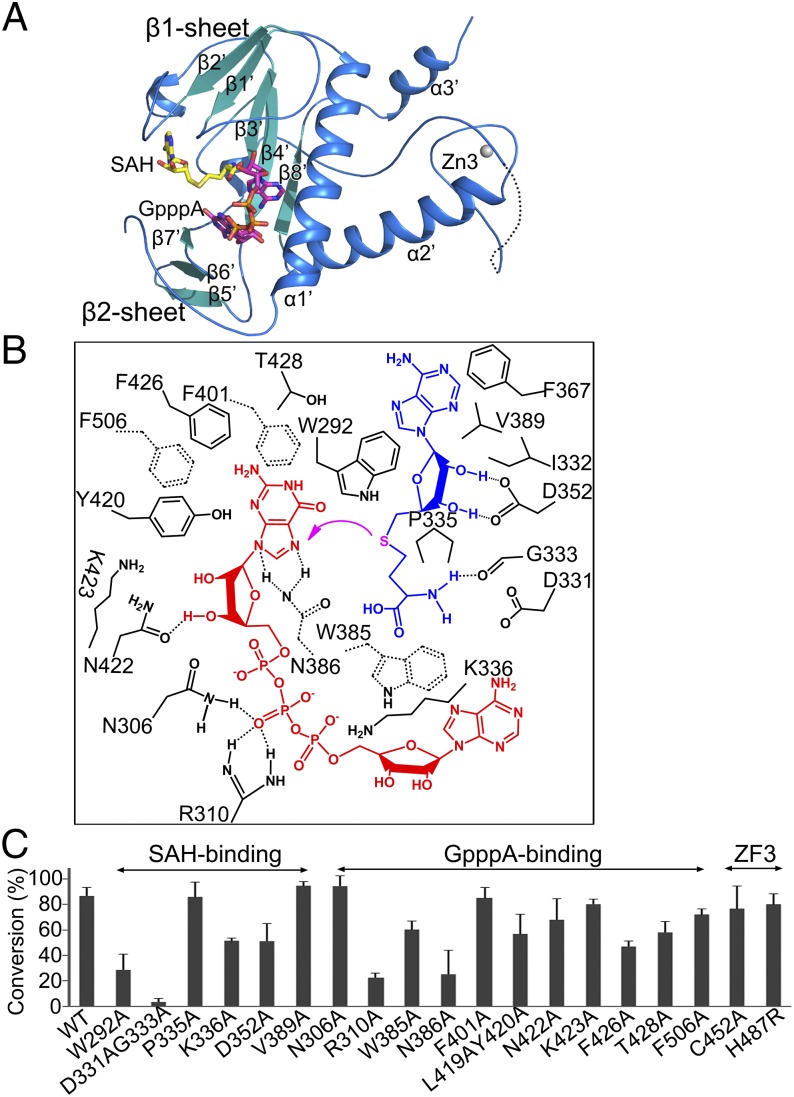
Fig. 4.
Structure and methyl transfer mechanism of the nsp14 N7-MTase domain. (A) Cartoon representation of the N7-MTase domain marked with secondary structural elements. (B) Amino acids within 4 Å of the ligands SAH (blue) and GpppA (red) are labeled and numbered. The magenta arrow indicates the methyl transfer. Dashed lines between residues indicate hydrogen bonds. Trp385, Asn386, Phe401, and Phe506 are shown by dashed bonds to depict their position below the plane of ligand GpppA. (C) The ability of nsp14 to methylate N7 of guanine of GpppA-RNA was measured. The results depict the efficiency of the conversion of substrate to product (%) and are plotted as a bar graph. WT nsp14 and its mutants were complexed with nsp10.