Figure 2.
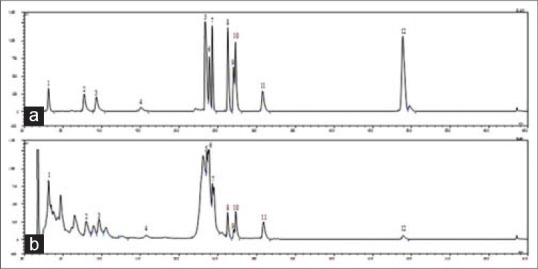
High-performance liquid chromatography-evaporative light scattering detector chromatograms of the mixture of standard compounds from Juglans sinensis (a) and Ethanolic extract of Juglans sinensis (b). The compounds were separated on on a Shiseido CapCell PAK C18 column (5 μm, 4.6 mm i.d. × 150 mm) using a periodic gradient solvent system with 20% B in 0–18 min, from 20% B to 60% B in 18–25 min, 60% B in 25–45 min and 85% B in 45–65 min. Solvent A: 0.1% formic acid, solven B: Acetonitrile. Flow rate: 1.0 mL/min. The peaks are (+)-catechin (1, tR 3.116 min), (+)-taxifolin 3-O-α-L-arabinofuranoside (2, tR 7.724 min), quercetin-3-O-α-L-arabinofuranoside (3, tR 9.288 min), kaempferol-3-O-α-L-arabinofuranoside (4, tR 15.079 min), quercetin (5, tR 23.424 min), 2α, 3β, 23-trihydroxy-olean-12-ene-28-oic acid 28-O-β-D-glucopyranoside (6, tR 23.875 min), 2α, 3α, 23-trihydroxy-urs-12-ene-28-oic acid 28-O-β-D-glucopyranoside (7, tR 24.294 min), arjunolic acid (8, tR 26.282 min), 1-oxo-3β, 23-dihydroxy-olean-12-en-28-oic acid (9, tR 27.049 min), 2α, 3α, 23-trihydroxy-urs-12-en-28-oic acid (10, tR 27.293 min), 23-hydroxyursolic acid (11, tR 30.826 min) and ursolic acid (12, tR 48.791 min)
