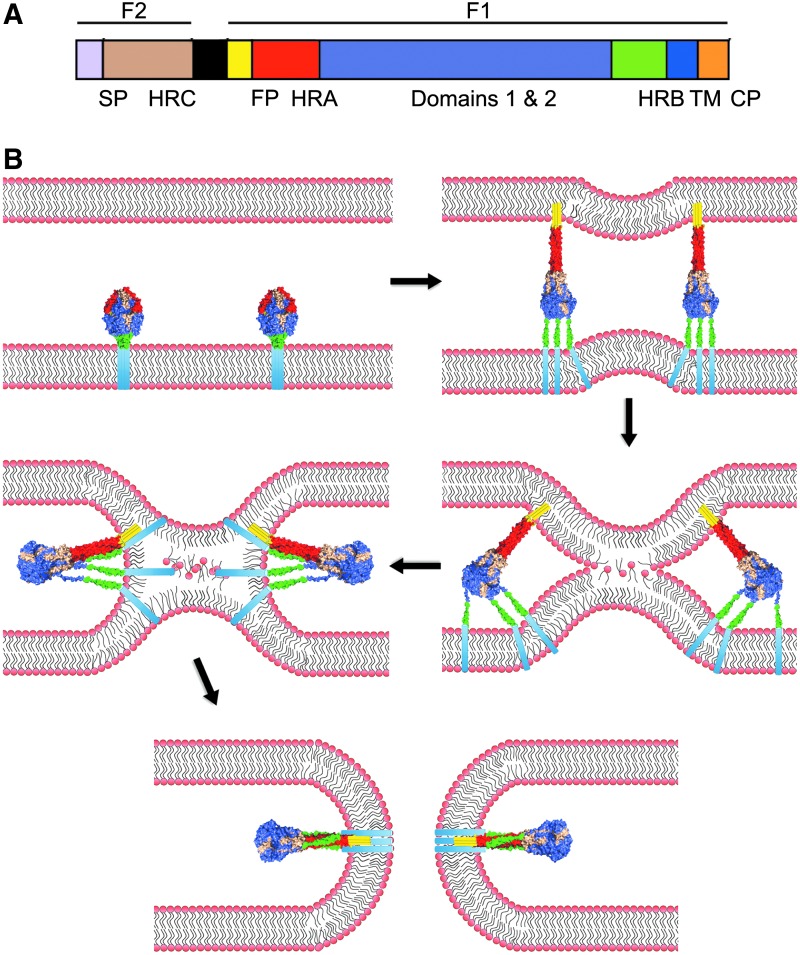
FIG. 1.
The respiratory syncytial virus (RSV) fusion machinery. (A) Schematic of the domain structure of the RSV fusion protein. RSV F is proteolytically cleaved into F1 and F2 subunits. The F1 subunit consists of the fusion peptide (FP; yellow), heptad-repeat A (HR-A; red), domains 1 and 2 (violet), heptad-repeat B (HR-B; green), a transmembrane domain (TM; blue), and the cytoplasmic tail (CP; orange). The F2 subunit consists of heptad-repeat C (HRC; tan). (B) For the viral entry, RSV F undergoes a series of conformational changes that result in the formation of the HR-A central trimeric coil and insertion of the fusion peptide into the target membrane. The HR-B domains then fold into the grooves of the HR-A triple helix, inducing local negative curvature in the membranes that promotes merger of the outer leaflets of the bilayers (hemifusion stage). Assembly of the 6HB leads to opening of a fusion pore. Color-coding of individual F domains as in (A).