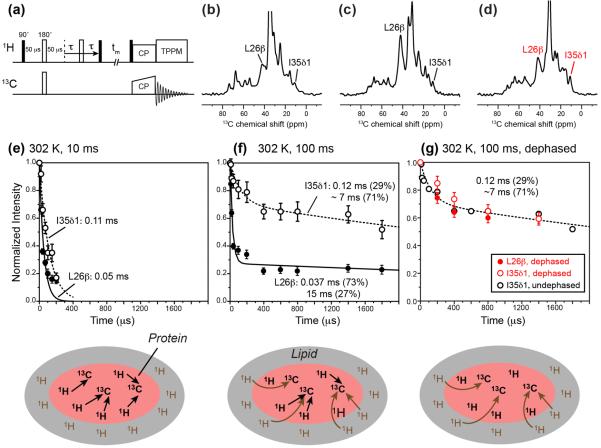
Figure 7.
13C-detected 1H T2 relaxation data of M2TM-AH in oriented bicelles at 302 K under the static condition. (a) Pulse sequence of the dipolar-dephased 1H T2 relaxation experiment used to obtain the data in (g). (b) Representative 13C spectra without dipolar dephasing, measured with 10 ms 1H spin diffusion. (c) Representative 13C spectra without dipolar dephasing, measured with 100 ms 1H spin diffusion. (d) Representative 13C spectra with dipolar dephasing and 100 ms 1H spin diffusion. The echo delay is 0 for all the spectra in (b–d). (e) 13C-detected 1H T2 relaxation curves with 10 ms spin diffusion. The schematic illustration below indicates that 1H magnetization transfer is mostly within the peptide at this mixing time. (f) 13C-detected 1H T2 relaxation curves after 100 ms spin diffusion. At this mixing time, lipid 1H magnetization from the surrounding environment is transferred to the peptide, thus giving much slower relaxation. A fast initial decay is especially pronounced for the L26 Cb signal. (g) 1H T2 relaxation curves measured with 100 μs C-H dipolar dephasing and 100 ms spin diffusion, to selectively detect the T2 relaxation of lipids near the peptide. The disappearance of the fast initial decay of the L26 Cb data verifies that the origin of the fast initial decay is the rigid peptide protons.