Abstract
Adults with burn scars are a clinical challenge, and the long term sequelae of burns can have a significant impact on the patient. Scar excision is thought to be the best treatment at present, as it results in a smaller scar. Scar stretching has shown promise in a previous study, as it may allow the surgeon to excise more burn scar. The goal of this study was to determine if good evidence exists for the use of burn scar stretching, in routine clinical practice, through the format of a critically appraised topic.
A question was formulated using the Patient Intervention Comparator Outcome (PICO) method:
-
–
Patient – Adult burn victims
-
–
Intervention – Scar excision + skin stretching
-
–
Comparator – Scar excision
-
–
Outcome – Total remaining scar
The PICO question was used to develop a search query: “stretch* burn scar” (where ‘*’ represents a wildcard function). A search was then conducted using PubMed, SCOPUS, the Cochrane Library, and Trip Database. One paper was selected for critical appraisal following identification, screening, and eligibility evaluation.
The paper was critically appraised using accepted methodology outlined by Straus et al. and reporting quality was assessed using the CONSORT statement for non-pharmacological trials.
Areas of methodological or reporting weakness were highlighted.
Burn scar stretching, using the device or technique in question, requires much further research before widespread usage in burns patients.
Keywords: Critically appraised topic, Burn scar, Skin stretching, Burn management, Randomized controlled trials, CONSORT
Introduction
“Evidence-based medicine (EBM) requires the integration of the best research evidence with our clinical expertise and our patient's unique values and circumstances.”1
Clinical Context
A burn is an acute traumatic injury to the skin by means of exposure to heat, cold, electrical, chemical, or radiation energy.2 Data from the United Kingdom (UK) National Burn Care Review (2001) estimates 250,000 cases of burn injuries annually, of which 90% are preventable. Of these, 175,000 present to Accident and Emergency departments with 13,000 requiring hospital admission, and 300 deaths.3
Most burns are relatively minor and can be safely treated in the community.4 Over the last 25 years, the chances of survival from significant burn injury have been steadily increasing owing to advances in critical care, nutrition, surgical protocols, and infection control.3 This situation requires a renewed focus regarding the control or rectification of chronic burn sequelae which can be detrimental to rehabilitation. The ultimate, or ideal, treatment goal is to return the individual to their preinjury state, and allow them to retake their place in society with unaltered potential.3 The scarring process that ensues following a burn can have devastating long-term functional and cosmetic outcomes. Pruritis, pain, and psychological morbidity are common and debilitating for the individuals involved.5
A multitude of reconstructive techniques have been described to improve burn scars, including: scar excision, tissue expansion, and various scar release techniques.6 Scar excision, followed by direct wound closure, gives the best outcome as it results in a smaller scar.7 However, large defect closure after burn scar excision can be challenging owing to high skin tension. Hence, it is often done as a multi-step procedure for larger burn scars.5 Mechanical skin stretching is a relatively new technique that is gaining increasing scrutiny, especially in the field of wound healing.8
The objective of this study was to determine if skin stretching is a useful intervention for burn scars by using a critically appraised topic method.
Method
Defining the research question
In order to conduct an evidence-based search, a Patient Intervention comparator Outcome (PICO) question was defined.9,10 This has been formulated below:
-
–
Patient – Adult burn victims
-
–
Intervention – Scar excision + skin stretching
-
–
Comparator – Scar excision
-
–
Outcome – Total remaining scar
Search Strategy
From the PICO question a search phrase was developed: stretch * burn scar. The Boolean operator “and” is assumed. The use of the asterisk results in alternate spellings for stretch at the point that the asterisk appears i.e. stretched, stretching, etc.
The search for evidence was rationalised using the 6S system to search for pre-appraised evidence (see Table 1).11 It should be noted that the American College of Surgeons (ACS) Surgery platform was not functioning at the time.
Table 1.
6S system sequential results
| 6S System | Sources Searched | Result |
|---|---|---|
| Systems: computerised decision support systems | No such system currently exists for burns | N/A |
| Summaries | – NICE Guidelines | |
| – UpToDate | ||
| – British Medical Journal | ||
| Clinical Evidence | ||
| Synopses of Syntheses | – DARE | No results |
| – UK DUETs | ||
| Syntheses: Systematic reviews | – Cochrane library | 52 results |
| – Trip database | ||
| Synopses of studies: evidence-based abstraction journals | – ACP Journal Club | No results |
| – Centre for Reviews and | ||
| Dissemination | ||
| Studies: original articles published in journals | – PubMed | 88 results |
| – Scopus |
Inclusion Criteria
The search was restricted to the English language. Only study designs such as systematic reviews and randomised controlled trials (RCTs) were considered. They are the criterion standard for minimising bias when evaluating healthcare interventions when properly designed, conducted, and reported.12 A preliminary search had already shown that one non-randomised study existed in the literature.13 In 2006, Melis et al. reported a prospective study documenting seven-year follow-up results, for scar width and patient satisfaction, following closure of various large tissue defects using a skin-stretching device.13 This study showed promising results, but there was no control group. As such, no comparison was made to the standard technique of serial scar excision and wound closure without skin stretching. This underscored the need to examine only RCTs and Systematic Reviews.
Exclusion Criteria
Non-English language and non-randomised studies were excluded. No date restrictions were imposed; the research and literature base for surgical burns management is known to be relatively small (PubMed and Scopus were searched back to 1948). Where an interim and final study existed for the same research, the interim study was excluded. Animal studies and purely cost-effectiveness studies were excluded.
Databases
The databases used to conduct the search included: PubMed, SCOPUS, the Cochrane Library, and the Trip Database. It was believed that these databases would provide all the relevant evidence in this area with collective access to over 40 million records. EMBASE and Medline were not searched as SCOPUS indexes over 18,000 journals and includes the journals that these databases cover.
Results
The search results are shown through a flow diagram (Figure 1).
Fig 1.
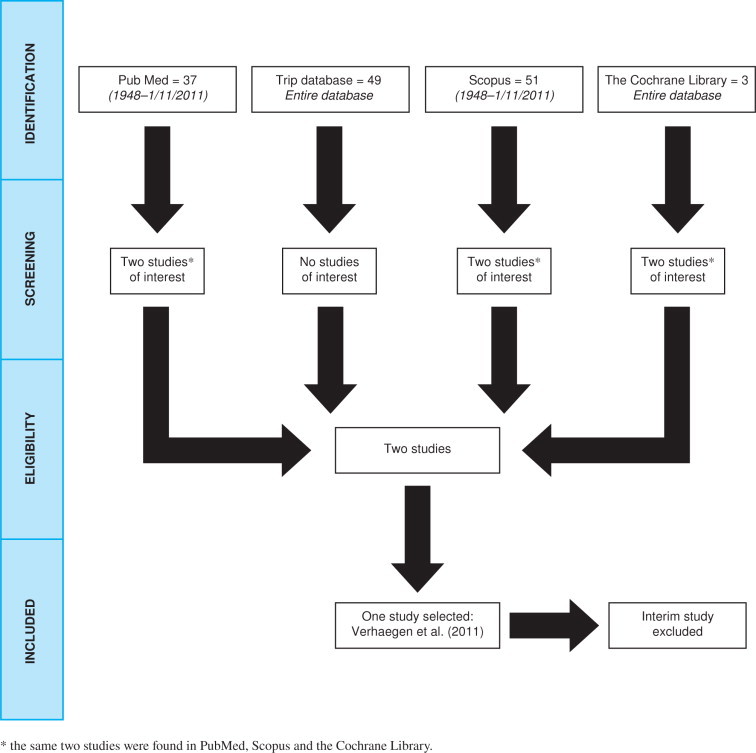
PRISMA Flow diagram illustrating the search results.
The Study Selected – Verhaegen et al. 2011 – Sustainable effect of skin stretching for burn scar excision: long-term results of a multicenter randomized controlled trial. A PICO table has been constructed to help summarise the paper (Table 2).
Table 2.
Summary table describing the Verhaegen et al. paper
| PICO Item | Details from the study |
|---|---|
| Patients | 30 patients with burn scars |
| Intervention | Scar excision + stretching (SS group) |
| Comparator | Scar excision alone (SE group) |
| Outcomes (at 12 months) | Objective |
| – Scar surface area measurements – using planimetry of sterile tracing sheets. | |
| – Scar colour – using Derma Spectrometer. | |
| Subjective | |
| – Patient and observer scar assessment scale (POSAS). | |
| – Scar hypertrophy. |
Critical Appraisal
In order to determine whether this treatment can be used with burns patients, first the validity, clinical relevance, and applicability of the results need to be assessed.
Is the research question an important and clearly focussed one?
Determining whether skin stretching could help burns patients is an important question and needs to be answered. This study does add to the literature as the first RCT to examine the potential for skin stretching for burn scar management.
Methodological Quality
To assess methodological quality, guidance provided by Straus et al. was used.1
Are the study participants representative of the target population?
From February 2008 until March 2010 thirty patients with burn scars were included (a pre-trial sample size and power calculation was either not performed or not reported). Details about the nature, context, and who performed the recruitment were not provided. Selection bias and regression to the mean effects could potentially be introduced by the study attracting those most desperate for a solution and thus willing to try new treatments. This would affect compliance and motivation and could result in Hawthorne effects in the patients under study.14 Indeed, Kirkley et al. showed how powerful Hawthorne effects can occur in surgical RCTs.15 The process of achieving informed consent for the participants is not detailed. Indeed, it would be important to understand how many potential participants rejected the new treatment option during the informed consent process.
Regarding the population specifically, the paper provides averages but not the ranges, medians, or standard deviations for patient age and scar age. The sex, ethnic group, social status, and co-morbidities of the patients recruited to the trial are not provided. Comorbidities such as depression are common in burn victims and this could represent an important confounder for the subjective outcomes.16 No mention is made of what previous procedures the patients have had to the same site, possibly to address the same problem(s). No mention is made of the anatomical sites involved, which areas of the body were burned, or the depth of burn. Also, the inclusion and exclusion criteria applied during the recruitment phase are not specified. Potential co-interventions standardised across the five trial centres is not made clear.
Was the assignment of patients to treatments randomised?
Whilst the paper has identified itself as an RCT at several points (title, introduction and method sections), there was no mention of how the randomisation process was actually performed (i.e. the method used to generate the allocation sequence, the type of randomisation, and whether blocking was used). Furthermore, there was no mention of the individuals who generated the random allocation sequence, enrolled participants, and assigned them to interventions.
Was the randomisation concealed?
Any steps taken to conceal the allocation sequence were not stated. Allocation concealment is a key marker of methodological quality in RCTs.17
Were the baseline characteristics for both groups similar at the start of the trial?
The process of randomisation should result in two balanced groups with an equivalent share of known and unknown confounders. It is, therefore, important to ensure that both groups are similar, in all ways important to prognosis, at the start of the trial. With proper randomisation baseline differences would be due to chance. If, by chance, the groups are not similar, then the need to adjustment for potentially important prognostic factors should be determined. The paper provides no table comparing both groups at the start of the trial. However, average ages and average scar ages were provided and the differences were not statistically significant.
Was the follow-up of patients sufficiently long and complete?
Patients were followed up at three and 12 months which is sufficiently long to determine outcomes. Twenty-nine out of 30 patients completed the 12 month follow-up period. However, one patient refused follow-up measurements at 12 months but no indication was given as to why. Thus, the loss to follow-up was relatively low at 3.33%. This is below the 5% threshold of the ‘5 and 20 rule’ i.e. less than 5% loss to follow-up leads to minimal bias and threat to validity.18
It is, however, not clear how many patients are in each group following randomisation. Whilst the paper mentions that only one patient was lost to follow-up, their results tables have n = 14 or n = 13 for each group, giving a sample size of 27 or 28 depending on the specific outcome (see Table 3). This conflict is not rationalised in the text.
Table 3.
Primary outcomes and results at 12 months
| Outcome Category | Measurement Made | Results at 12 months |
||
|---|---|---|---|---|
| SS Group | SE Group (control) | p-value | ||
| Scar surface area measurements | Total remaining scar area | 26% (n = 13) 95% CI not provided |
43% (n = 14) 95% CI not provided |
0.026 |
| Scar hypertrophy | Linear scarring | 21% (n = 13) 95% CI not provided |
25% (n = 14) 95% CI not provided |
0.607 |
| Scar Colour | Erythema | 5.70 (n = 14) 95% CI 0.50–10.13 |
6.68 (n = not stated) 95% CI 1.03–12.17 |
0.513 |
| Melanin | 4.93 (n = 14) 95% CI 0.20–23.20 |
4.56 (n = not stated) 95% CI 0.10–15.10 |
0.727 | |
| Mean POSAS Score | Patient | 3.9 (n = 14) 95% CI 1.5–6.5 |
3.9 (n = not stated) 95% CI 1.5–7.7 |
0.760 |
| Clinician | 3.6 (n = 14) 95% CI 2.4–7.0 |
3.5 (n = not stated) 95% CI 1.8–5.5 |
0.462 | |
Were all patients analysed in the groups to which they were randomised?
No mention is made of whether an intention-to-treat or protocol-based analysis was performed. Such analysis preserves the value and fidelity of randomisation. In addition, no mention of the degree of cross-over between treatment and control arms is mentioned. No worst-case or sensitivity analysis was performed, but given the loss to follow-up of just 3.33%, there is unlikely to be a large impact on the results. However, all patients were accounted for at the trial's conclusion. An indication of the compliance of the patients is not detailed.
Were patients, clinicians and study personnel kept blind to treatment?
There was no mention of blinding for three of the four outcomes measures. Scar hypertrophy was scored from pictures by a plastic surgeon experienced in burn reconstructions, who was not involved in this study and ‘blinded’ to prevent bias. Many of the measurements are subjective and hence lack of blinding could have introduced differential measurement error and measurement bias.19 The ‘objective’ outcome of scar surface area measurements involved tracing around the scar with sterile tracing sheets at 12 months. The individual doing this could have been blinded to the patient's allocation in the trial – although this was not mentioned. The person doing this at the 12-month follow-up need not have been a member of the same team. Greater use of blinding for assessors, patients, and indeed surgeons would have helped ensure greater reliability for the study.
Were groups treated equally, apart from the experimental therapy?
This is not well detailed within the trial report. This is a multicentre trial and it's unclear how the treatment was standardised across the five centres involved. The level of expertise of the people involved and where they are on the relevant learning curve for delivering this novel treatment is unknown. Did any issues of therapeutic equipoise come into play – especially for those assigned to perform the standard treatment and who were not blinded? There is no detail or pre-published protocol on whether any co-treatments were involved and how they were standardised across all the centres (e.g. psychological, anaesthetic, analgesic and nutritional support or regimes which could have an impact on the subjective outcomes). Little information was provided on background on the institutes involved. How many, and what type of burns patients they treat per year, and what facilities are available? Who performed the treatment and who did the aftercare?
This also affects the external validity of the work as other units would not be able to gauge if they could deliver this as part of their burns service. Furthermore, with only 30 patients in total, each centre contributed a relatively small number of patients making their individual results prone to type II error.
Was there an appropriate measurement of outcomes?
The POSAS score used in the study, is a composite score from the surgeon and the patient. Such composite outcomes must be assessed cautiously.20 Greenhalgh argues that measuring pain and other symptomatic effects is fraught with problems and outcome measures must be objectively validated.21 The POSAS score was validated in 2005 when 100 linear surgical scars were assessed by three independent observers with good inter- and intra-observer reliability.22
Whilst the study does present mean values for the primary outcomes it would have been more appropriate to provide median values which would have been more robust against extreme values, especially since no evidence to suggest a normal distribution within the study population is presented.
What is the magnitude of the treatment effect and how precise was it?
The main outcomes and results for the study are shown below together with p-values and 95% confidence intervals (95% CI).
Table 3 shows that the only statistically significant outcome was total remaining scar percentage; 26% in the SS group against 43% in the SE group (p = 0.026). However, no 95% CIs for the individual groups were provided. The authors do provide a 95% CI of 2–31 but this figure seems to be used for the group as a whole (both SS and SE groups) and doesn’t allow us to estimate the precision of the treatment effect for each group. 95% CI were not provided for the linear scarring outcome. In addition, the number of participants in some of the groups was not provided.
Reporting Quality
The Consolidated Statement on the reporting of non-pharmacological trials (CONSORT NPT) published in 2008 was used to determine the reporting quality of the paper.23 The paper scored 13 out of 23 mandatory reporting items (Table 4 in supplementary data online, a completed CONSORT NPT checklist taken directly from paper by Boutron et al. 200823).
Importantly, the paper was non-compliant in the areas of:
-
–
Randomisation details
-
–
Allocation concealment
-
–
Blinding
-
–
Flow diagram of participants
These areas have been found to be lacking in a previous study of the reporting quality of 160 RCTs in Surgery,24 and is now increasingly recognised as a problem within the surgical literature.25,26,27
Other Reporting Concerns
The paper does mention that there were no conflicts of interest and that they received funding from the Dutch Burns Foundation. No mention is made of ethical approval for the study and the relevant ethical committee's judgement reference, or whether methodology and handling of participants was in line with the Declaration of Helsinki. With the aim of increasing the transparency in research, the International Committee of Medical Journal Editors (ICMJE) made it mandatory for every RCT to be registered prior to outset;28 however, no such registration number was provided.
The implications for the patient with burns – clinical relevance and applicability
Defining the appropriate population, and the injury patterns, where this treatment would be superior are key to determining clinical applicability. All clinically important outcomes were not considered, e.g. quality of life 12 months post-procedure would have been very useful (using validated instruments like the Hamilton method or EQ-5D).28,29 It would be important to understand how much of a biological, or clinical, impact the skin-stretching device provides versus the control. Statistical significance may not reliably translate to biological significance or patient satisfaction.
Assessing the applicability of the burn stretching treatment for burns patients in the English National Health Service also requires an understanding of whether the potential benefits are worth the harms and costs. With respect to harms, the study details a section on complications which included reoperations for scar releases (one patient in SE group) and dog ear corrections (one patient in SS group). However, no mention is made of how much the new treatment and the control compare in terms of cost. In addition, there was no mention of how operating times or length-of-stay differed, or how easy it was to obtain the relevant stretching devices.
Limitations
The limitations of this CAT include restriction to English language only studies, and the exclusion of non-randomised studies.
Conclusion
One of the values of an RCT is its potential to be incorporated into systematic reviews once a critical mass of studies has been reached. However, accurate replication necessitates that comprehensive information has been provided in research studies.30 RCTs in surgery are difficult to initiate and conduct well.31 To use Paul Glasziou's analogy, it is difficult to determine whether this RCT had a fair start, fair race, and fair finish.1 So how does the EBM practitioner reconcile the promise of such a new treatment (if only in the area of total remaining scar %). The sensible strategy, at this point in time, is to call for more research, stimulate debate and discussion in the field, and to thank the authors for their contribution.
Ethical approval
No ethical approval required for this study.
Conflict of interest
No conflicts of interest have been declared by the author.
Author contribution
Single author manuscript.
Funding
No funding source declared by author.
Open Access: This article is published Open Access at annalsjournal.com. It is distributed under the AMS terms and conditions, which permits unrestricted non commercial use, distribution, and reproduction in any medium, provided the original authors and source are credited.
Footnotes
Provenance and Peer Review
Unsolicited, externally peer-reviewed
References
- 1.Straus S.E., Glasziou P., Richardson W.S., Haynes R.B. 4th. Churchill Livingstone; Toronto: 2011. Evidence-Based Medicine: How to practice and teach it. [Google Scholar]
- 2.Rice PL and Orgill DP. Classification of burns. UpToDate 2011 [Online] Available from: http://www.uptodate.com/contents/classification-of-burns?source=search_result&search=burn&selectedTitle=2~150 (accessed 10 July 2012).
- 3.National Burn Care Review Committee. National burn care review, 2001. [online] Available from: http://www.britishburnassociation.org/downloads/NBCR2001.pdf (Accessed 10 July 2012).
- 4.Hudspith J., Rayatt S. ABC of burns: first aid and treatment of minor burns. BMJ. 2004;328(7454):1487–1489. doi: 10.1136/bmj.328.7454.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shelley O.P., Dziewulski P. Late management of burns. Surgery. 2006;24:15–17. [Google Scholar]
- 6.Barret J.P. Burns reconstruction. BMJ. 2004;329:274. doi: 10.1136/bmj.329.7460.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verhaegen P.D., van der Wal M.B., Bloemen M.C., Dokter J., Melis P., Middelkoop E., van Zuijlen P.P. Sustainable effect of skin stretching for burn scar excision: long-term results of a multicenter randomized controlled trial. Burns. 2011;37(7):1222–1228. doi: 10.1016/j.burns.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Agha R., Ogawa R., Pietramaggiori G., Orgill D. A Review of the Role of Mechanical Forces in Cutaneous Wound Healing. Journal of Surgical Research. 2011;171:700–708. doi: 10.1016/j.jss.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Richardson W.S., Wilson M.C., Nishikawa J., Hayward R.S. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123(3):A12–A13. [PubMed] [Google Scholar]
- 10.Oxman A.D., Sackett D.L., Guyatt G.H. Users’ guides to the medical literature. I. How to get started. The Evidence-Based Medicine Working Group. JAMA. 1993;270(17):2093–2095. [PubMed] [Google Scholar]
- 11.DiCenso A., Bayley L., Haynes R.B. Accessing preappraised evidence: fine-tuning the 5S model into a 6S model. Ann Intern Med. 2009;151(6) doi: 10.7326/0003-4819-151-6-200909150-02002. JC3–2, JC3–3. [DOI] [PubMed] [Google Scholar]
- 12.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melis P., van Noorden C.J., van der Horst C.M. Long-term results of wounds closed under a significant amount of tension. Plastic and Reconstructive Surgery. 2006;1:259–265. doi: 10.1097/01.prs.0000195080.65662.f5. [DOI] [PubMed] [Google Scholar]
- 14.McCarney R., Warner J., Iliffe S., van Haselen R., Griffin M., Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkley A., Birmingham T.B., Litchfield R.B., Giffin J.R., Willits K.R., Wong C.J., Feagan B.G., Donner A., Griffin S.H., D’Ascanio L.M., Pope J.E., Fowler P.J. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359(11):1097–1107. doi: 10.1056/NEJMoa0708333. [DOI] [PubMed] [Google Scholar]
- 16.Tuker P. Psychosocial problems among adult burn victims. Burns. 1987;13(1):7–14. doi: 10.1016/0305-4179(87)90249-x. [DOI] [PubMed] [Google Scholar]
- 17.Linde K., Ramirez G., Mulrow C.D., Pauls A., Weidenhammer W., Melchart D. St John's wort for depression-an overview and meta-analysis of randomised clinical trials. BMJ. 1996;313:253–258. doi: 10.1136/bmj.313.7052.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardarelli R., Oberdorfer J.R. Evidence-Based Medicine. Part 5. An Introduction to Critical Appraisal of Articles on Prognosis. J Am Osteopath Assoc. 2007;107(8):315–319. [PubMed] [Google Scholar]
- 19.Franks P. Clinical trials. Fam Med. 1998;20(6):443–448. [PubMed] [Google Scholar]
- 20.Ferreira-González I., Permanyer-Miralda G., Busse J.W., Bryant D.M., Montori V.M., Alonso-Coello P., Walter S.D., Guyatt G.H. Methodologic discussions for using and interpreting composite endpoints are limited, but still identify major concerns. J Clin Epidemiol. 2007;60(7):651–657. doi: 10.1016/j.jclinepi.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Greenhalgh T. Assessing the methodological quality of published papers. BMJ. 1997;315(7103):305–308. doi: 10.1136/bmj.315.7103.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Kar A.L., Corion L.U., Smeulders M.J., Draaijers L.J., van der Horst C.M., van Zuijlen P.P. Reliable and feasible evaluation of linear scars by the Patient and Observer Scar Assessment Scale. Plast Reconstr Surg. 2005;116(2):514–522. doi: 10.1097/01.prs.0000172982.43599.d6. [DOI] [PubMed] [Google Scholar]
- 23.Boutron I., Moher D., Altman D.G., Schulz K.F., Ravaud P. Methods and processes of the CONSORT Group: example of an extension for trials assessing nonpharmacologic treatments. Ann Intern Med. 2008;148(4):W60–W66. doi: 10.7326/0003-4819-148-4-200802190-00008-w1. [DOI] [PubMed] [Google Scholar]
- 24.Agha R., Cooper D., Muir G. The reporting quality of randomised controlled trials in surgery: A systematic review. International Journal of Surgery. 2007;5(6):413–422. doi: 10.1016/j.ijsu.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Camm CF, Agha RA and Edison E. CONSORT Adherence in Journals is Still Far from Perfect. Annals of Surgery 2012 (article in press). [DOI] [PubMed]
- 26.Edison E., Agha R., Camm C.F. Poor quality reporting and surgical RCTs: A wake up call for the international surgical community. Spine. 2012;37(7):628. doi: 10.1097/BRS.0b013e318247f3f9. [DOI] [PubMed] [Google Scholar]
- 27.Edison E., Agha R., Camm C. No excuse for poor reporting of surgical RCTs. Journal of Orthodontics. 2011;38:305–307. doi: 10.1179/14653121141605. [DOI] [PubMed] [Google Scholar]
- 28.Torrance G.W. Utility approach to measuring health-related quality of life. Journal of Chronic Diseases. 1987;40(6):593–600. doi: 10.1016/0021-9681(87)90019-1. [DOI] [PubMed] [Google Scholar]
- 29.EuroQol Group. EQ-5D [online]. Available at: http://www.euroqol.org/home.html (accessed 10 July 2012).
- 30.Glasziou P., Meats E., Heneghan C., Shepperd S. What is missing from descriptions of treatment in trials and reviews? BMJ. 2008;336(7659):1472–1474. doi: 10.1136/bmj.39590.732037.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCulloch P., Taylor I., Sasako M., Lovett B., Griffin D. Randomised trials in surgery: problems and possible solutions. BMJ. 2002;324(7351):1448–1451. doi: 10.1136/bmj.324.7351.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]


