Abstract
Information forwarded by individual muscle spindles is modulated by the dynamic and static gamma motoneurons in a differentiated way, depending on the coupling between the fusimotor neurons and the various intrafusal muscle fibres. Further modulation of this information at the level of spinal neurons is also differentiated because connections between individual muscle spindles and their spinal target cells are quite variable. This review illustrates this variability with respect to the spinal trajectory of muscle spindle primary afferents and the distribution of their synaptic contacts on motoneurons and other spinal neurons. It also discusses some of the consequences of this variability for the processing of information from proprioceptors.
Keywords: interneurons, motoneurons, muscle spindle afferents, spinal cord
Introduction
The accuracy of the information on muscle length reaching spinal neurons may depend on several factors; not only on how this information is obtained by muscle spindles but also on how it is transferred to the spinal cord. Both specificity and variability, and even randomness, have been found to characterize the connections between muscle spindles and spinal neurons – connections between fusimotor gamma motoneurons and intrafusal muscle fibres in one direction, and between primary and secondary afferent endings in muscle spindles and their spinal target cells in the other. The aim of this review is to draw attention to this variability and to some of its functional consequences.
Variability in fusimotor innervation
Morphological studies revealed that bag 1 intrafusal muscle fibres are innervated exclusively by dynamic gamma motoneurons, while both bag 2 and chain intrafusal fibres are under the control of static as well as dynamic gamma motoneurons in different combinations (see the review by Banks RW 2015 in this issue). As summarized by Banks (1981, 1994, 2015), individual gamma motoneurons may innervate only a single or a number of intrafusal muscle fibres, either alone or together with several other gamma motoneurons. In addition, they may innervate intrafusal muscle fibres at only one or at both poles of a spindle and at different distances from the sensory terminals. As a consequence of the variability of the sites at which the intrafusal muscle fibres are innervated, as illustrated in Fig.1a,b, focal contractions of these muscle fibres are evoked by gamma motoneurons at a range of distances from the equatorial region of the muscle spindles. This was demonstrated by stimulation of single fusimotor nerve fibres and direct observations of their effects in single intrafusal muscle fibres (Banks et al. 1978); the range of distribution of the focal contractions evoked in this way (Fig.1c) corresponded well to the range of areas within which the intrafusal fibres are innervated by fusimotor nerve fibres (Fig.1a,b).
Fig 1.
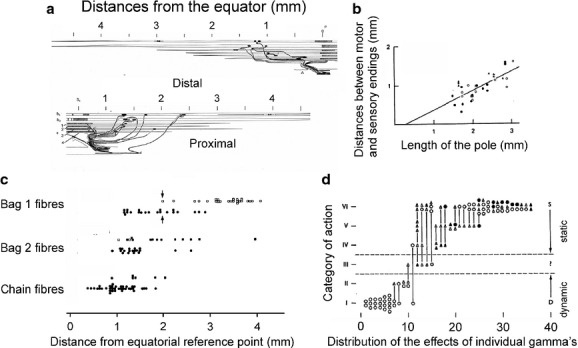
Contacts and actions of feline fusimotor neurons. (a) Reconstruction of contacts of fusimotor fibres innervating the two poles of a muscle spindle illustrating the range of distances (approximately 1.5–2.5 mm) from the equator at which these contacts were formed. (b) Plot of distances between the centres of the motor and sensory endings (ordinate) against the length of the intrafusal muscle fibre pole, taking the centre of the primary ending as the equatorial limit, illustrating an even larger range of distances from the equator. (c) Distribution of foci of contractions evoked by single fusimotor fibres from the equatorial reference point. (d) The range of action exerted by a sample of 37 feline individual fusimotor fibres on different primary endings. The strongest static actions correspond to category VI and the strongest dynamic actions to category I, the symbols indicating fusimotor actions on two–five primary endings. (a and b) Modified from figs 1 and 5 in Banks (1981); (c) from fig. 2 in Banks et al. (1978); (d) from fig. 14 in Emonet-Denand et al. (1977). In this and in the following figures, all reproductions are with permission.
This variability is further expressed in differences between actions of individual gamma motoneurons on various muscle spindles, and by combinations of joint actions of dynamic and static gamma motoneurons on these spindles. By comparing effects of single gamma motoneurons on a number of muscle spindles in the same muscle, Emonet-Denand et al. (1977) showed for instance that these effects may vary in strength, and be more static or more dynamic (Fig.1d). The situation was summarized by Banks by describing muscle spindles and their innervation by fusimotor neurons as “a system constructed by an interplay of deterministic and random factors” (Banks, 1994).
Thus, in order to extract as accurate as possible information about muscle stretches and contractions, spinal neurons cannot rely on signals from single muscle spindles. They may, however, use a strategy of integrating information from several muscle spindles, by using a summary or an average of the incoming signals. The extreme case of such summation occurs in spinal motoneurons, as individual motoneurons are contacted by group Ia afferents from the great majority of muscle spindles in a muscle (Mendell & Henneman, 1971). To integrate information from several muscle spindles becomes even more vital in view of the variability of connections between individual muscle spindle afferents and their spinal target cells, which is even more marked than that between gamma motoneurons and the spindles. A great number of potential combinations of contacts of primary and secondary muscle spindle afferents on various target cells, originating from either the same or from different muscles, further adds to the interplay of the deterministic and random factors in the formation of these connections.
Variability in projections and distribution of contacts between muscle spindle primaries and spinal neurons
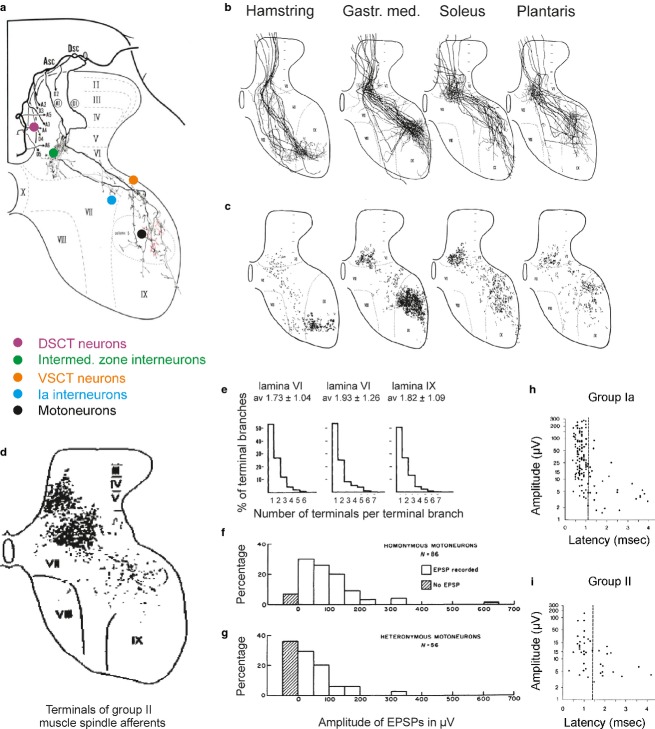
Considerable differences were found in both the intraspinal trajectory and the projection areas of individual primary afferent fibres. This was not only in the case of fibres from different spindles, but also of axon collaterals of single fibres and in the number of synaptic contacts that they form with their target cells. These differences were revealed by intra-axonal labelling of feline muscle spindle primary afferents (group Ia afferents according to the terminology used in physiological studies) and subsequent reconstructions. As illustrated in Fig.2a with reconstructions made by Ishizuka et al. (1979), individual intraspinal collaterals of a single group Ia afferent traverse different parts of the spinal grey matter and give off terminal axonal branches within somewhat different areas. Even more marked differences characterize projections and distribution of terminal contacts of populations of these afferents, whether from the same or other muscles (Fig.2b,c). Differences were also found in the number of synaptic contacts formed by the various terminal axonal branches (Fig.2e).
Fig 2.
Variability of projections and distribution of terminals of muscle spindle afferents. (a) Intraspinal trajectory of axon collaterals of a muscle spindle primary afferent from the medial gastrocnemius intra-axonally labelled with HRP. Locations of five populations of target cells contacted by terminal axonal branches of this afferent are indicated by coloured circles. (b and c) Superimposed reconstructions of similarly intra-axonally labelled primaries from four muscles (as indicated above), and of the distribution of their axon collaterals and terminals. (d) Distribution of terminals of muscle spindle secondaries. The dots represent terminals of intra-axonally labelled functionally identified afferents from hindlimb muscles in the cat. (e) Histograms of numbers of axon terminals per terminal branch in various laminae. (f and g) Distribution of amplitudes of excitatory postsynaptic potentials (EPSPs) evoked in homonymous and heteronymous motoneurons by single primary muscle spindle afferents. (h and i) Amplitude-latency scatter diagrams for EPSPs evoked by single group Ia and group II muscle spindle afferents. (a–c and e) From figs 2, 6, 8 and 9 in Ishizuka et al. (1979); (d) from fig. 1 in Hongo (1992); (f and g) from fig. 5 in Mendell & Henneman (1971). DSCT, dorsal spinocerebellar tract; VSCT, ventral spinocerebellar tract. (h and i) from fig. 4 in Watt et al. (1976) and from fig. 4 in Stauffer et al. (1976).
A further expression of the variability and randomness of synaptic contacts formed by the group Ia afferents is the distribution of these contacts along dendrites of individual motoneurons (Fig.3). This was analysed using two supplementary experimental approaches. The first of these was to establish contacts between pairs of individual intracellularly labelled feline group Ia afferents and motoneurons innervating the same muscle, as illustrated in Fig.3g,h for the soleus muscle and its afferents. Contacts were formed within relatively narrow domains traversed by dorso-laterally running regularly spaced axon collaterals of the afferents, on a restricted number of dendrites and at greatly varying distances from the soma. The contacts are indicated by filled or open circles in the wiring diagram in Fig.3f, and by open circles in Fig. 3h; for details and for the quantitative analysis see Burke & Glenn (1996). The second approach was to reconstruct contacts on individual similarly intracellularly labelled rat motoneurons, but using an antibody against VGLUT1 to label contacts from all of the group Ia afferents onto these motoneurons, as illustrated in Fig.3d,e, with quantitative details in Rotterman et al. (2014). The results were comparable, demonstrating contacts on only some of the dendritic branches, at different distances from the soma and at varying densities.
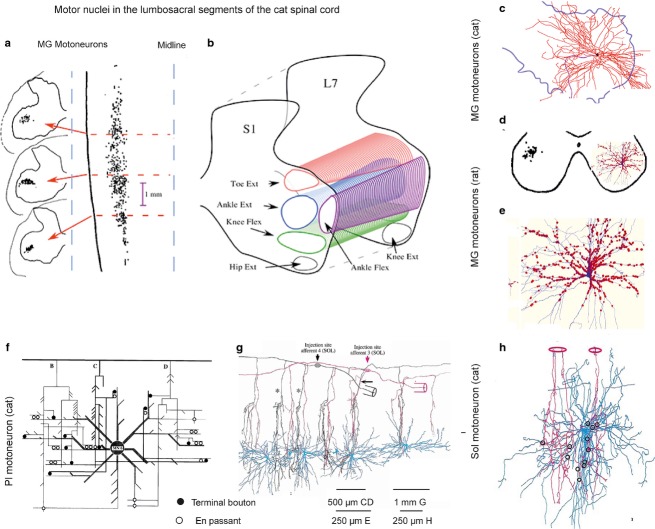
Fig 3.
Synaptic contacts between muscle spindle primaries and motoneurons. (a) Retrogradely labelled cell bodies of feline medial gastrocnemius (MG) motoneurons in three transverse planes (left) and in a horizontal plane (right). (b) Columns of feline hindlimb motor nuclei in a transverse plane. (c and d) Dendritic trees of two MG motoneurons overlying most of the ventral horn area in the cat and the rat, respectively. (e) Expanded dendritic tree of the motoneuron in (d) (in blue) with contacts of VGLUT1-IR terminals (red) representing contacts of muscle spindle afferents, mainly primaries but perhaps also secondaries. These are the only peripheral afferents synapsing with alpha motoneurons. (f) Wiring diagram of contacts between three adjacent feline plantaris (Pl) afferent collaterals and dendrites of a Pl motoneuron. (g) Reconstruction of the trajectory of axon collaterals of two feline soleus Ia afferents crossing dendritic trees of six motoneurons at different distances from their cell bodies in a sagittal plane. (h) Reconstruction of contacts of two feline soleus afferent collaterals with a soleus (Sol) motoneuron. (a–c) From figs 1 and 3 in Burke (2013); (d, e) from fig. 4 in Rotterman et al. (2014); (f–h) from figs 1, 2 and 8 in Burke & Glenn (1996).
However, despite the randomness of distribution of afferent contacts within their projection areas, terminals of axonal branches of all analysed muscle spindle group Ia afferents were found in the five areas indicated in Fig.2a. These areas are within the motor nuclei, in a strip just outside the motor nuclei, in lamina VII, in laminae V and VI, and in Clarke’s column. They correspond to the areas of location of five main populations of spinal target cells of the primaries: motoneurons; Ia inhibitory interneurons; ventral spinocerebellar tract neurons; intermediate zone interneurons; and dorsal spinocerebellar tract neurons, respectively, as indicated in Fig.2a. Information from the same muscle spindles may thus be distributed to samples of all of these neuronal populations. There is also an order behind the variable projections of muscle spindle primary afferents, as afferents from different muscles have preferred target cells, at least in motor nuclei (Fig.2c). They also converge on several populations of spinal neurons following preferred patterns of convergence, thereby ensuring the purposely determined actions of the afferents. For instance, Ia inhibitory interneurons are contacted by afferents of as restricted an origin as motoneurons, while intermediate zone interneurons are contacted both by muscle spindle primaries from a variety of muscles, and by muscle spindle secondary afferents and tendon organ afferents from either the same or different muscles. These relationships were identified first in the cat, but appear to apply to primates, including humans, as well as to mice.
Because of their preferred projections to particular motor nuclei, primary afferents evoke largest compound excitatory postsynaptic potentials (EPSPs) in motoneurons innervating the same (homonymous) muscle, while smaller EPSPs are evoked in motoneurons of synergists, even though amplitudes and central latencies of EPSPs evoked in individual motoneurons vary (Fig.2f–h; Eccles & Lundberg, 1958; Mendell & Henneman, 1971; Watt et al. 1976). The specificity of these connections is further evidenced by the fact that they are formed not only within the motor nuclei, where cell bodies of motoneurons innervating various muscles are located within long narrow spatially separated columns (Fig.3a,b), but also in the area outside motor nuclei, where dendrites of motoneurons from different nuclei are intermingled because the dendritic trees cover several motor nuclei within a 1–2 mm radius. This is illustrated in Fig.3, both with a representative dendritic tree of an individual feline adult motoneuron (Fig.3c) and by comparing the extent of this dendritic tree with the extent of motor nuclei in the transverse plane in Fig.3b (in the same scale). A similar situation in the rat is illustrated in Fig.3d with the dendritic tree of a motoneuron (right) extending outside the area of its motor nucleus (left), and has also been found for ensembles of motoneurons in a motor pool in neonatal mice preparations (Vrieseling & Arber, 2006). As synaptic contacts of the primary afferents have been found along the whole dendritic length, this indicates these contacts are formed on dendrites of homonymous motoneurons outside their nuclei, but not on dendrites of other motoneurons with which they overlap.
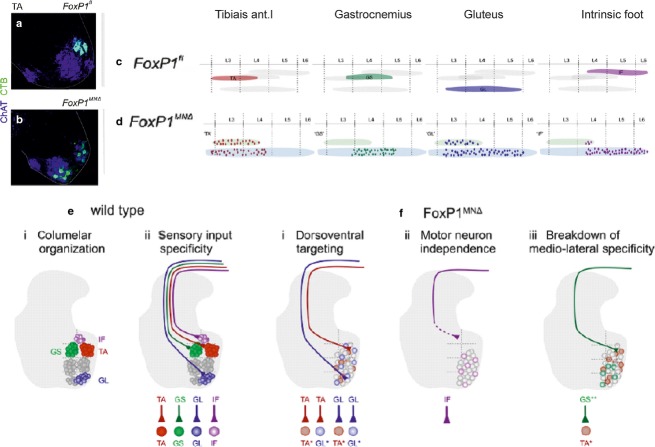
How the selective and specific connections between muscle spindle primary afferents and motoneurons are formed remained unresolved for many years. While they were linked with several factors, recent studies of Surmeli et al. (2011) revealed a particular importance of the location of columns of motoneuron pools and of their clustering. Connections between the afferents and motoneurons were analysed in a genetically modified mouse preparation under two conditions: either with a preserved location of the columns (Fig.4a,c,e); or after the motoneurons were displaced by inactivation of a motoneuron transcriptional cofactor for Hox proteins, FoxP1, which blocks Hox output and strips embryonic motoneurons of their distinctive pool identities and cadherin profiles and disrupts their clustering and location (Fig.4b,d,f). The results summarized in Fig.4e,f led to the conclusion that the projection area of the afferents and not the attraction by specific motoneurons is the main factor determining formation of synaptic contacts with any motoneurons within this area.
Fig 4.
Changes in the connectivity between group Ia afferents and motoneurons related to the dislocation of the motoneurons. (a and b) Positions of tibialis anterior (TA) motoneurons labelled by retrograde transport of cholera toxin B (CTB, light green) in a wild-type mouse preparation and in a mouse mutant in which FoxP1 protein was absent in motoneurons. (c and d) As in (a and b), but showing rostrocaudal distribution of four motoneuron pools. (e) Diagrams of motor columns in wild-type mice and of selective coupling between group Ia afferents and homonymous motoneurons. (f) Diagrams illustrating breakdown in specific connections between Ia afferents and motoneurons after the dislocation of motor nuclei in the FoxP1 mutant. Modified from figs 2, 4 and 7 in Surmeli et al. (2011). ChAT, choline acetyltransferase; GL, gluteus; GS, gastrocnemius; IF, intrinsic foot.
Factors determining the terminal projection areas of the afferents in the FoxP1 mutant have not been fully established, but one may consider that the original target cells of the primary afferents in these preparations include not only motoneurons but also Ia interneurons and spinocerebellar neurons with similar group Ia input (Ishizuka et al. 1979; see Fig.2a). Cells of these other populations are apparently not stripped of their molecular identities (Surmeli et al. 2011) and might therefore remain as targets for the same afferents. No information is as yet available for contacts between the primary afferents and the dendrites of the displaced motoneurons in the same mice mutant, except that in the analysed preparations the contacts were found on the somata and the most proximal, but not distal, parts of the dendrites (Surmeli et al. 2011).
The connectivity between muscle spindle primary afferents and different populations of spinal interneurons might be similarly determined by the overlapping areas of location of the interneurons and of the terminal projection areas of the afferents. As proposed by Surmeli et al. (2011, p. 662)…. “one virtue of relying on a connectivity logic based on position is that it permits sensory afferents to engage, coordinately, the many interneuron subtypes allocated to the firing of a single motor pool, without the molecular burden of allocating matching surface labels to each contributing neuronal type” … The connectivity based on spatial factors could nevertheless involve afferents with both narrow and wide terminal projection areas and interneuronal populations located within very small as well as large parts of the spinal grey matter, targeting motoneurons within only a single or several motor pools. Both electrophysiological and morphological studies indicate that individual interneurons in spinal reflex pathways have a great variety of target cells, including motoneurons, other interneurons and ascending tract cells. These studies show also that interneurons belonging to various functional populations do not form distinct nuclei, but are distributed within fairly large areas where they are intermingled with interneurons of other populations. This has been demonstrated for interneurons with afferent input from selected muscles labelled by trans-ganglionic transport in the mouse by Zampieri et al. (2014), as well as with retrograde trans-synaptic transport from motoneurons of WGA-HRP in the cat (Harrison et al. 1984; Alstermark & Kummel, 1986), or viruses in the mouse (Stepien et al. 2010). Mice interneurons of the same embryonic origin likewise appear to be characterized by a widespread distribution over several Rexed laminae (Al-Mosawie et al. 2007; Dougherty & Kiehn, 2010; Zhang et al. 2014). Furthermore, the current data show fairly large areas of distribution of all premotor interneurons in the cat, including those targeting a very limited number of motor nuclei and with a very restricted afferent input, for example, Ia inhibitory interneurons. In addition, even if cell bodies of Ia inhibitory interneurons are located relatively close to motor nuclei, as shown in Fig.5a, the dendritic trees of these interneurons extend far from the cell body (Fig.1b) and cover a great part of the ventral horn.
Fig 5.
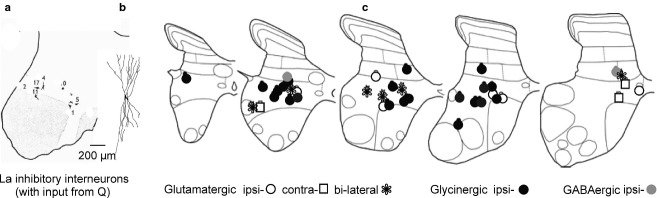
Distribution of functionally identified populations of interneurons. (a) Location of cell bodies of a sample of Ia inhibitory interneurons with input from the quadriceps muscles in the 6th lumbar segment of the cat; they were labelled by intracellularly injected HRP. (b) Reconstruction of the dendritic tree of one of the interneurons of this population. (c) Location of a sample of feline intermediate zone premotor interneurons sharing input from group I and/or group II afferents from the quadriceps, but found to be either excitatory or inhibitory (defined by the transmitter content in their terminals) and to have axons projecting ipsilaterally, bilaterally or contralaterally. All these interneurons were antidromically activated from the gastrocnemius or hamstring motor nuclei, and were all labelled by intracellularly applied HRP. (a and b) From figs 2 and 6 in Jankowska & Lindstrom (1972); (c) from fig. 5 in Bannatyne et al. (2009). Q, quadriceps.
The areas of location of premotor interneurons within the intermediate zone (laminae IV–VI) are even larger than of Ia inhibitory interneurons, especially if one considers the extent of their dendritic trees (Figs6a and 7d–g). Figure5b exemplifies this with the location of somata of a sample of feline interneurons with afferent input from group I and/or II muscle spindle afferents, having as their target cells the gastrocnemius-soleus or hamstring motor nuclei at the border between the 7th lumbar and 1st sacral segments.
Fig 6.
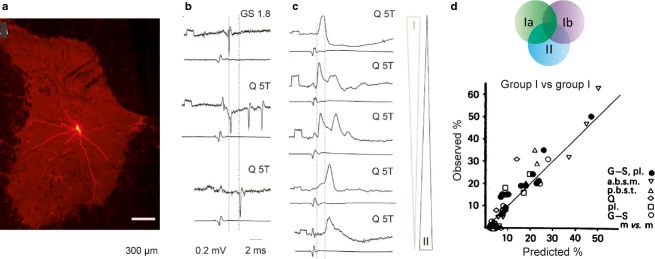
Distribution of input from different sources to feline intermediate zone premotor interneurons. (a) An example of the extent of dendrites of intermediate zone interneurons (rhodamine dextran-labelled interneuron). (b) Examples of extracellular records of responses from three interneurons, evoked by group I, group I and II, and group II afferents, respectively. (c) A series of intracellular records illustrating combinations of EPSPs of different amplitudes evoked from group I and II afferents in five interneurons. (d) A diagram illustrating a roughly linear relationship between the observed frequency of input from various combinations of input and frequencies expected on the basis of random connections of afferents with overlapping terminal projection areas. (a) Modified from fig. 2 in Liu et al. (2010); (b and c) from fig. 1 in Jankowska & Edgley (2010); (d) modified from fig. 3 in Harrison & Jankowska (1985). GS, gastrocnemius-soleus; Q, quadriceps; T, threshold.
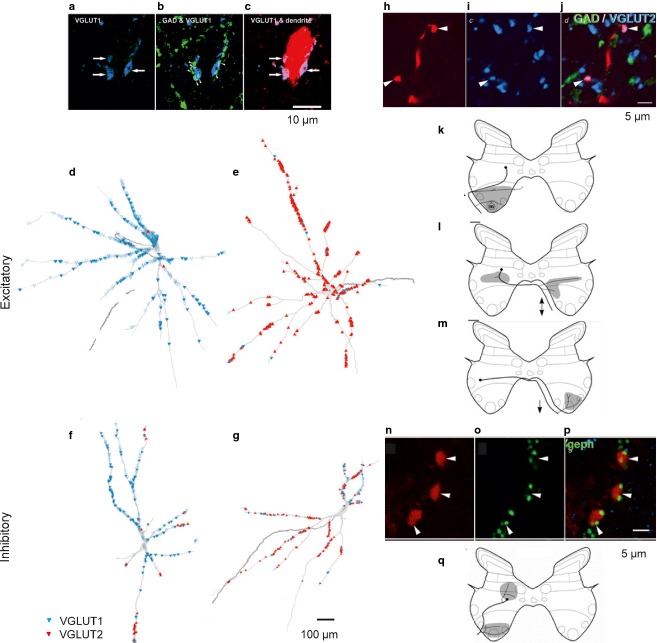
Fig 7.
Heterogenous character of feline intermediate zone premotor interneurons with input from muscle spindle afferents based on the analysis of immunochemistry of axon terminals synapsing with them, their phenotype and axonal projection. (a–c) Characteristics of terminals (blue, glutamatergic) forming contacts with an interneuron labelled by intracellular injection of rhodamine dextran (red); single (a,b) and merged (c) confocal images, with green showing GAD (GABAergic) immunofluorescence. (d–g) Reconstruction of the distribution of VGLUT1 (d,f) and VGLUT2 (e,g) terminals on two excitatory (d,e) and two inhibitory (f,g) interneurons. (h–j) Transmitter characteristics of terminals of excitatory interneurons labelled with rhodamine dextran (red) revealed by antibodies against VGLUT2. (k–m) Examples of axonal projections of three subpopulations of excitatory interneurons. (n–p) Transmission characteristics of terminals of inhibitory interneurons labelled with rhodamine dextran (red) revealed their association with gephyrin immunoreactivity (green – another marker of inhibitory synapses). (n) An example of exclusively ipsilateral axonal projections of inhibitory interneurons. (a–g) and (h–q) From figs 1, 2, 6 and 7 in Bannatyne et al. (2009), and from figs 2 and 5 in Liu et al. (2010). GAD, glutamic acid dehydrogenase (a marker for inhibitory neurons); VGLUT, vesicular glutamate transporter.
Variability in patterns of joint actions of muscle spindle afferents and of other sources of input to spinal interneurons
Intermediate zone interneurons with input from both muscle spindle primary and secondary afferents and from tendon organs are particularly interesting from the point of view of the variability of these input patterns. The results of combined electrophysiological, morphological and immunohistochemical studies in the cat have shown that this population is highly heterogenous, even though they process information from generally the same pool of afferents. They make contacts with a variety of other neurons in addition to motoneurons, including both excitatory and inhibitory interneurons with ipsilateral, bilateral or contralateral axonal projections, and thereby of apparently different embryonic origin. However, as indicated by different symbols in Fig.5c, subpopulations of interneurons of different phenotypes or with different axonal projections may be located side by side, and appear to be as a rule intermingled. The total distribution of these interneurons coincides with the terminal projection areas of group Ia afferents from a number of muscles (Fig.2c), as well as of group II afferents (Fig.2d), group Ib tendon organ afferents and some other, for example, joint, afferents. The interneurons may thus integrate information from different combinations of these afferents. However, no distinct subpopulation of these interneurons has been distinguished based on their input patterns. On the contrary, all samples of feline intermediate zone interneurons appear to be characterized by a distributed input from muscle spindle afferents. As illustrated in Fig.6c, they show a continuum of input from either group Ia or group II afferents, from very small to large EPSPs in intracellular records, and combinations of EPSPs of different amplitudes from different muscles. Extracellular records show that the resulting action potentials may be evoked from either group Ia afferents, both group Ia and II afferents, or only group II afferents (Fig.6b), but would depend on the combinations of sub-threshold and supra-threshold EPSPs and of the level of depolarization of these neurons by other sources of input.
Three kinds of experiments were made to evaluate factors determining the patterns of convergence upon these interneurons. Firstly, the number of afferent fibres needed to activate them was estimated from the sizes of unitary EPSPs evoked by graded electrical stimulation of a peripheral nerve or by a muscle stretch, and by the number of unitary EPSPs needed to induce an action potential. These estimates indicated that only a few muscle spindle afferents from a number of muscles may provide information to individual interneurons (Lundberg et al. 1975), in contrast to a near totality of muscle spindle group Ia afferents in a single muscle that form synaptic contacts with their target alpha motoneurons (Mendell & Henneman, 1971; Watt et al. 1976), though with a smaller proportion of muscle spindle group II afferents (Stauffer et al. 1976). Individual interneurons are thus apparently involved in processing only small samples of the information that is forwarded to motoneurons but from larger receptive fields.
Using another approach, it was examined whether individual interneurons receive information from preferred combinations of afferents, for example, from two or three particular muscles, or from muscle spindle afferents with or without tendon organs, or else whether any afferent fibres terminating within an area randomly make contacts with neurons in this area. The probability of random combinations was accordingly compared with the actual frequencies of these combinations (as illustrated in Fig.6d), and the results greatly favoured random patterns of convergence on individual interneurons (Harrison & Jankowska, 1985; Edgley, 2001).Nevertheless, the outcome of input to individual interneurons forwarded at random can be pooled together, or averaged, due to the convergent actions of several interneurons on their target motoneurons.
The third approach used to analyse patterns of these contacts was to visualize them on intracellularly labelled feline interneurons by using immunocytochemistry for vesicular glutamate transporters (VGLUTs), a marker for excitatory, glutamatergic, transmission. The distribution of either VGLUT1 or VGLUT2 terminals (Fig.7d–g) generally supported a randomized contact formation as they only covered some dendritic branches and as their density varied greatly. However, while entirely random connections between peripheral afferents and interneurons located within the afferent terminal projection areas would make it likely that different numbers of afferent terminals form synaptic contacts with the interneurons, it would be unlikely for some of the interneurons to fail to be contacted by the afferents. Thus, the finding that a significant proportion of intermediate zone interneurons practically lack contacts with VGLUT1-containing terminals might indicate that these contacts are actually not formed quite randomly, and that some additional factors determine whether the interneurons are contacted predominantly by the afferents or by other sources of input with VGLUT2 in their terminals (Liu et al. 2010). In addition, a mixed VGLUT1 and VGLUT2 input was found to characterize most of the intermediate zone interneurons, but some showed an almost exclusive VGLUT1 or VGLUT2 input (Liu et al. 2010), with examples in Fig.7d–g.
To compare patterns of convergence and actions of muscle spindle and other afferents on subpopulations of intermediate zone interneurons in detail would require a combination of techniques that are hard to use while analysing individual interneurons. These are: electrophysiological techniques, including intracellular recording to define the peripheral input; immunohistochemical techniques combined with labelling of functionally or genetically identified interneurons to differentiate between the excitatory and inhibitory ones and/or those of different embryonic origin; and morphological techniques to characterize their axonal projections. So far, combinations of only some of these techniques have been applied. However, they have failed to provide indications of a distinctly different distribution of input to subpopulations of excitatory and inhibitory feline interneurons (Liu et al. 2010), including those with unilateral, bilateral or contralateral axonal projection (Bannatyne et al. 2009), or to subpopulations of mice V2 interneurons (Al-Mosawie et al. 2007; Dougherty & Kiehn, 2010; Zhang et al. 2014), at least some of which correspond to the subpopulations of feline intermediate zone interneurons with input from group I and/or II afferents.
Concluding remarks on functional consequences of the variability of connections between muscle spindle afferents and spinal neurons
As mentioned above, the variability in connections between muscle spindle afferents and spinal neurons may to some extent be compensated by combined actions of smaller or larger proportions of these afferents on their spinal target cells. By pooling information from several muscle spindles, spinal neurons may thus be able to extract more accurate information on the degree of muscle stretches or contractions and on their dynamic characteristics. However, the internal organization of spinal neuronal networks and their supraspinal control does not seem to be developed primarily to assist in this task. On the contrary, it appears to be directed towards some particular aspects of this information, either by enhancing or by reducing it, depending on the behavioural context. For instance, very weak synaptic actions of either muscle spindle primary or secondary afferents may be used to discharge their target cells when assisted by convergent input from other sources. In contrast, excessively strong actions of these afferents under other circumstances may be weakened by presynaptic inhibition or by actions of various groups of inhibitory interneurons. It may thus be advantageous for synaptic actions of muscle afferents not to be too strong, so that these actions may be both enhanced or reduced, depending on the demands of a given situation.
Acknowledgments
The work done in the author’s laboratory was supported by the National Institutes of Health (R01 NS040863) and the Swedish Research Council (VR). The collaboration with Drs D.J. Maxwell and I. Hammar and their comments, as well as Dr Bewick’s comments on this review are gratefully acknowledged.
References
- Al-Mosawie A, Wilson JM, Brownstone RM. Heterogeneity of V2-derived interneurons in the adult mouse spinal cord. Eur J Neurosci. 2007;26:3003–3015. doi: 10.1111/j.1460-9568.2007.05907.x. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Kummel H. Transneuronal labelling of neurones projecting to forelimb motoneurones in cats performing different movements. Brain Res. 1986;376:387–391. doi: 10.1016/0006-8993(86)90205-2. [DOI] [PubMed] [Google Scholar]
- Banks RW. A histological study of the motor innervation of the cat’s muscle spindle. J Anat. 1981;133:571–591. [PMC free article] [PubMed] [Google Scholar]
- Banks RW. The motor innervation of mammalian muscle spindles. Prog Neurobiol. 1994;43:323–362. doi: 10.1016/0301-0082(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Banks RW, Barker D, Bessou P, et al. Histological analysis of cat muscle spindles following direct observation of the effects of stimulating dynamic and static motor axons. J Physiol (Lond) 1978;283:605–619. doi: 10.1113/jphysiol.1978.sp012522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks RW. The innervation of the muscle spindle: a personal history. J Anat (in this issue) 2015 doi: 10.1111/joa.12297. DOI: 10.1111/joa.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Liu TT, Hammar I, et al. Excitatory and inhibitory intermediate zone interneurons in pathways from feline group I and II afferents: differences in axonal projections and input. J Physiol (Lond) 2009;587:379–399. doi: 10.1113/jphysiol.2008.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE. Spinal motoneurons. In: Pfaff D, editor. Neuroscience in the 21st Century. New York: Springer Verlag; 2013. pp. 1027–1062. [Google Scholar]
- Burke RE, Glenn LL. Horseradish peroxidase study of the spatial and electrotonic distribution of group Ia synapses on type-identified ankle extensor motoneurons in the cat. J Comp Neurol. 1996;372:465–485. doi: 10.1002/(SICI)1096-9861(19960826)372:3<465::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Dougherty KJ, Kiehn O. Functional organization of V2a-related locomotor circuits in the rodent spinal cord. Ann N Y Acad Sci. 2010;1198:85–93. doi: 10.1111/j.1749-6632.2010.05502.x. [DOI] [PubMed] [Google Scholar]
- Eccles RM, Lundberg A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol (Lond) 1958;144:271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA. Organisation of inputs to spinal interneurone populations. J Physiol. 2001;533:51–56. doi: 10.1111/j.1469-7793.2001.0051b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet-Denand F, Laporte Y, Matthews PB, et al. On the subdivision of static and dynamic fusimotor actions on the primary ending of the cat muscle spindle. J Physiol (London) 1977;268:827–861. doi: 10.1113/jphysiol.1977.sp011884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Hultborn H, Jankowska E, et al. Labelling of interneurones by retrograde transsynaptic transport of horseradish peroxidase from motoneurones in rats and cats. Neurosci Letters. 1984;45:15–19. doi: 10.1016/0304-3940(84)90322-7. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E. Organization of input to the interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol (Lond) 1985;361:403–418. doi: 10.1113/jphysiol.1985.sp015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T. Patterns of spinal projection of muscle spindle group II fibres. In: Jami L, Pierrot-Deseilligny E, Zytnicki D, editors. Muscle Afferents and Spinal Control of Movement. Oxford: Pergamon Press; 1992. pp. 389–394. [Google Scholar]
- Ishizuka N, Mannen H, Hongo T, et al. Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: three dimensional reconstructions from serial sections. J Comp Neurol. 1979;186:189–211. doi: 10.1002/cne.901860206. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update. Eur J Neurosci. 2010;32:881–893. doi: 10.1111/j.1460-9568.2010.07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Lindstrom S. Morphology of interneurones mediating Ia reciprocal inhibition of motoneurones in the spinal cord of the cat. J Physiol (Lond) 1972;226:805–823. doi: 10.1113/jphysiol.1972.sp010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Bannatyne BA, Jankowska E, et al. Properties of axon terminals contacting intermediate zone excitatory and inhibitory premotor interneurons with monosynaptic input from group I and II muscle afferents. J Physiol (Lond) 2010;588:4217–4233. doi: 10.1113/jphysiol.2010.192211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Convergence from Ib, cutaneous and joint afferents in reflex pathways to motoneurones. Brain Res. 1975;87:81–84. doi: 10.1016/0006-8993(75)90783-0. [DOI] [PubMed] [Google Scholar]
- Mendell LM, Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971;34:171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Rotterman TM, Nardelli P, Cope TC, et al. Normal distribution of VGLUT1 synapses on spinal motoneuron dendrites and their reorganization after nerve injury. J Neurosci. 2014;34:3475–3492. doi: 10.1523/JNEUROSCI.4768-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer EK, Watt DG, Taylor A, et al. Analysis of muscle receptor connections by spike-triggered averaging. 2. Spindle group II afferents. J Neurophysiol. 1976;39:1393–1402. doi: 10.1152/jn.1976.39.6.1393. [DOI] [PubMed] [Google Scholar]
- Stepien AE, Tripodi M, Arber S. Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron. 2010;68:456–472. doi: 10.1016/j.neuron.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Surmeli G, Akay T, Ippolito GC, et al. Patterns of spinal sensory-motor connectivity prescribed by a dorsoventral positional template. Cell. 2011;147:653–665. doi: 10.1016/j.cell.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieseling E, Arber S. Target-induced transcriptional control of dendritic patterning and connectivity in motor neurons by the ETS gene Pea3. Cell. 2006;127:1439–1452. doi: 10.1016/j.cell.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Watt DG, Stauffer EK, Taylor A, et al. Analysis of muscle receptor connections by spike-triggered averaging. 1. Spindle primary and tendon organ afferents. J Neurophysiol. 1976;39:1375–1392. doi: 10.1152/jn.1976.39.6.1375. [DOI] [PubMed] [Google Scholar]
- Zampieri N, Jessell TM, Murray AJ. Mapping sensory circuits by anterograde transsynaptic transfer of recombinant rabies virus. Neuron. 2014;81:766–778. doi: 10.1016/j.neuron.2013.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lanuza GM, Britz O, et al. V1 and v2b interneurons secure the alternating flexor-extensor motor activity mice require for limbed locomotion. Neuron. 2014;82:138–150. doi: 10.1016/j.neuron.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]