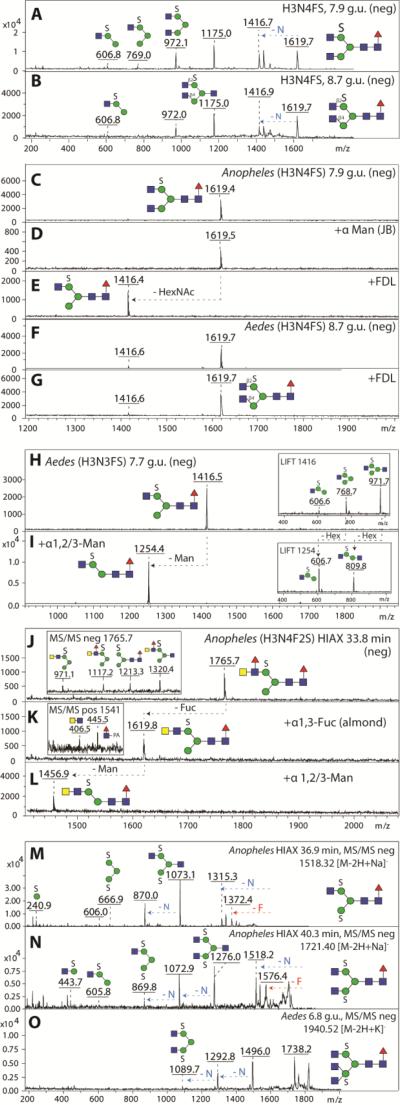
Figure 6. Elucidation of sulphated N-glycan structures in negative ion mode MS.
MS/MS of isomeric biantennary sulphated glycans (H3N4FS, m/z 1619) eluting at 7.9 (A) and 8.7 g.u. (B) are rather similar, but showed different susceptibility to β1,2-specific FDL hexosaminidase. The earlier eluting isomer of Anopheles (C) is resistant to jack bean α-mannosidase (D), and sensitive to β1,2-specific FDL (E), whereas the later eluting isomer predominantly found in Aedes (F) remains resistant to β1,2-specific FDL treatment (G) suggesting a β1,4-linked GlcNAc residue on the α1,3-mannose arm. A sulphated pseudohybrid glycan species of Aedes (H3N3FS, m/z 1416, H) is sensitive towards α-1,2/3-specific mannosidase treatment (I) thereby reducing the MS/MS key fragments by Δm/z = 162 (insets). A glycan (H3N4F2S, m/z 1765, J) in an Anopheles HIAX fraction displays MS/MS key fragments indicating the presence of a fucosylated LacdiNAc motif in addition to sulphated mannose(inset in J) and loses one fucose upon treatment with α1,3-fucosidase (K) and one mannose upon subsequent α1,2/3-mannosidase treatment (L). Positive ion mode MS/MS upon α1,3-fucosidase indicated the presence of core fucose (inset in K). MS/MS of disulphated N-glycans of the compositions H3N3FS2 (M), H3N4FS2 (N) and H3N5FS2 (O) are compatible with sulphation of both α-mannose residues; a further indication for this position is that these glycans were sensitive to jack bean β-hexosaminidase, but resistant to jack bean α-mannosidase.