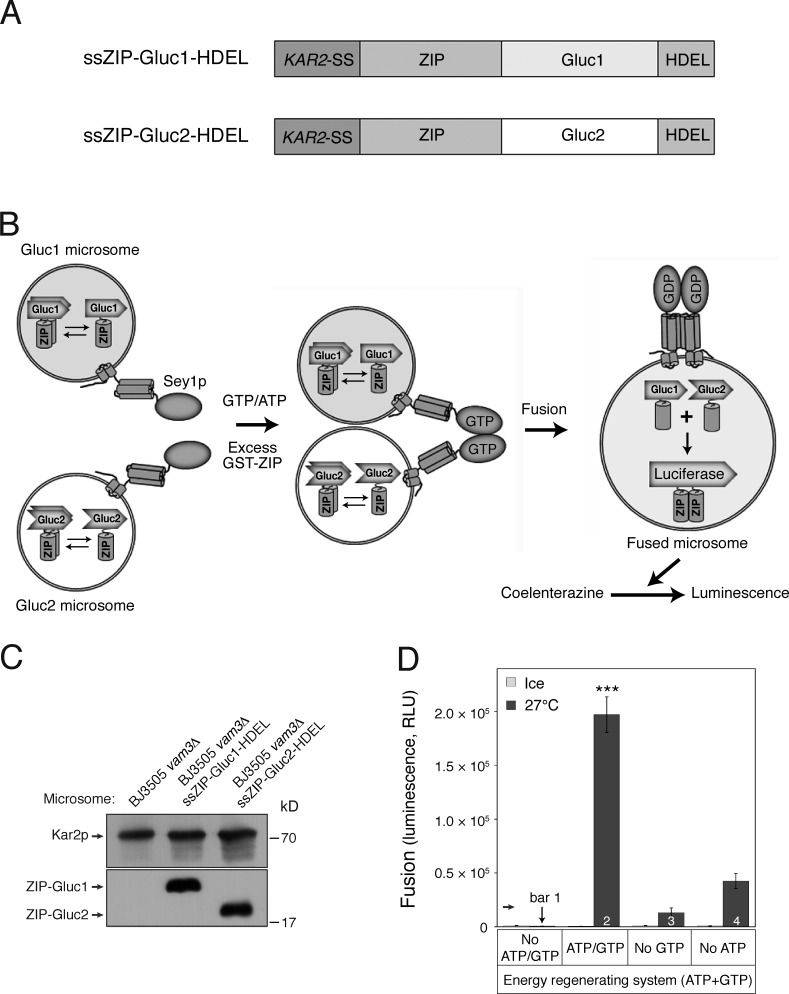
Figure 1.
Development of an in vitro homotypic ER fusion assay. (A) Schematic representation of chimeric proteins for the Gluc PCA. (B) Assay scheme; see Results for details. Microsomes isolated from BJ-Gluc1 yeast cells overexpressing ssZIP-Gluc1-HDEL under the control of an ADH1 promoter (Gluc1 microsomes) were mixed with microsomes isolated from BJ-Gluc2 yeast cells overexpressing ssZIP-Gluc2-HDEL (Gluc2 microsomes) and then incubated at 27°C in the presence of GTP and ATP. After 90 min, the luciferase substrate coelenterazine was added, and luciferase activity was measured. Excess GST-ZIP was added to block extra-luminal reconstitution of functional Gluc caused by membrane destabilization or rupture during incubation. (C) Expression of Gluc PCA fragments in isolated microsomes. The expression of Gluc PCA fragments was analyzed by immunoblotting using the indicated antibodies. Kar2p, an ER-resident protein, was used as a loading control. (D) GTP-driven homotypic ER fusion is markedly enhanced by ATP. Gluc1 and Gluc2 microsomes were mixed and incubated on ice or at 27°C in the presence of ATP and/or GTP for 90 min. Data represent means ± SEM (error bars; n = 3). RLU, relative luminescence unit. ***, P < 0.001, Tukey’s test between experiments performed at 27°C.