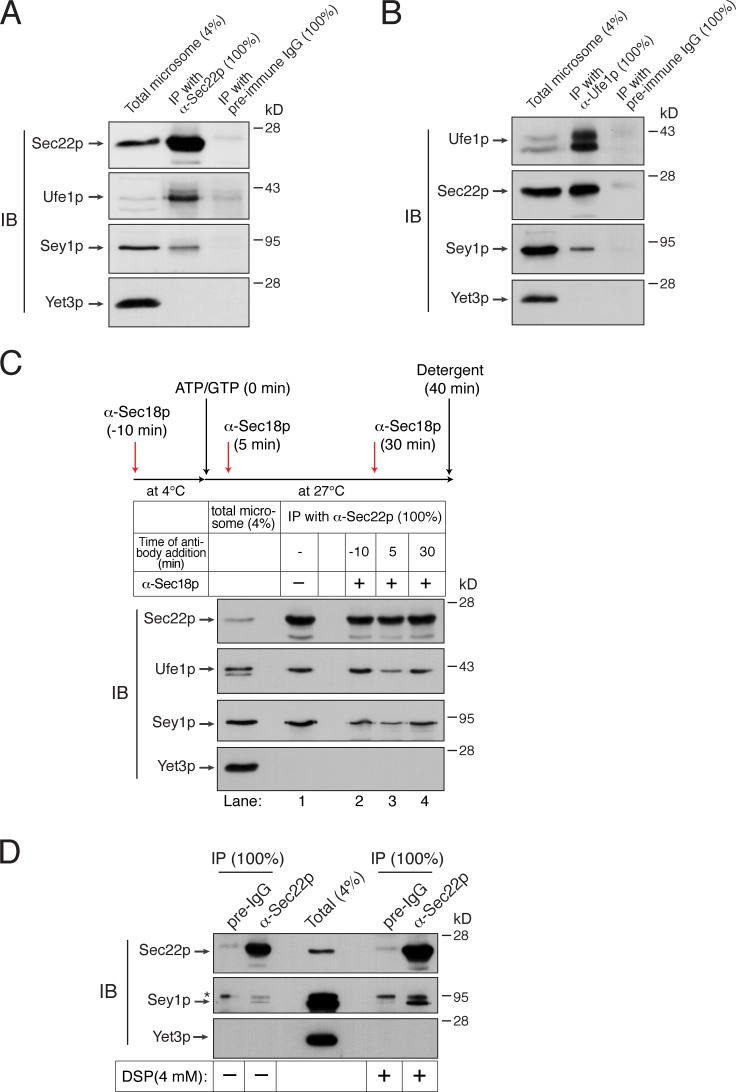
Figure 6.
Sey1p physically interacts with ER SNAREs. (A and B) Sey1p physically interacts with the ER SNARE proteins Sec22p and Ufe1p. Microsomes isolated from BJ3505 were detergent-solubilized and incubated with anti-Sec22p antibodies (A), anti-Ufe1p antibodies (B), or preimmune IgG (control) in the presence of protein A Sepharose. Protein A Sepharose–bound material was then analyzed by immunoblotting using the indicated antibodies. (C) Sec18p regulates the interaction between Sec22p and Sey1p. BJ3505 microsomes were preincubated in the absence or presence of anti-Sec18p antibodies for 10 min at 4°C. After ATP/GTP was added, microsomes were further incubated for 40 min. During incubation, some samples received anti-Sec18p antibodies at the indicated time points. Sec22p was precipitated using anti-Sec22p antibody–conjugated Dynabeads, and bound proteins were analyzed by immunoblotting using the indicated antibodies. (D) Detection of an in vivo interaction between Sec22p and Sey1p by DSP cross-linking. BJ3505 spheroplasts were incubated in the absence or presence of 4 mM DSP for 30 min at 4°C. After DSP was quenched, detergent-solubilized spheroplasts were subjected to immunoprecipitation using anti-Sec22p antibodies or preimmune IgG (preIgG). Cross-links were cleaved using β-mercaptoethanol in SDS sample buffer, and proteins cross-linked with Sec22p were analyzed by immunoblotting using the indicated antibodies. The asterisk indicates nonspecific bands. All experiments were performed multiple times with similar results, and the data shown are representative of all results.