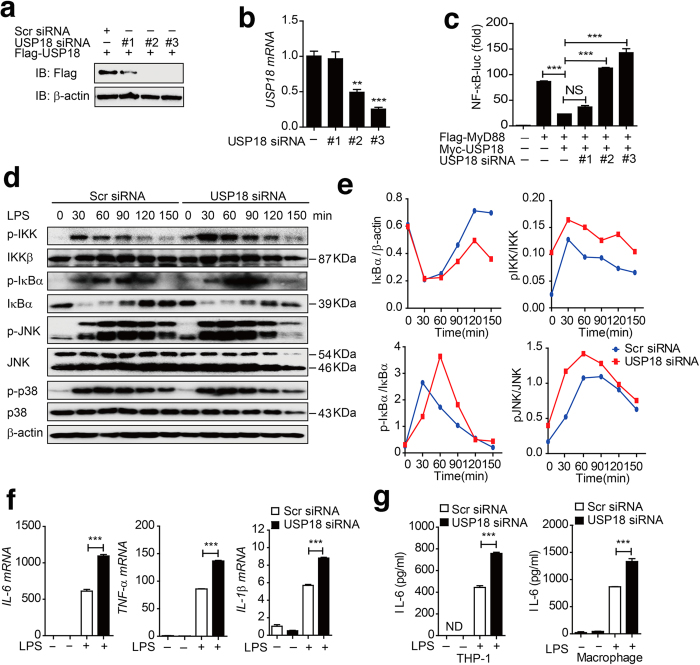
Figure 2. Knockdown of USP18 enhances NF-κB activation as well as the inflammatory response.
(a) The knockdown efficiency of USP18-specific siRNA. Immunoblot analysis of the knockdown of exogenous USP18 in HEK293T cells expressing Flag-USP18 or endogenous USP18 in THP-1 cells treated with USP18-specific siRNA or scrambled (Scr) siRNA. β-actin serves as a loading control. (b) The knockdown efficiency of USP18-specific siRNA at the mRNA level. Real-time PCR analysis of endogenous USP18 in HEK293T cells treated with USP18-specific siRNA or scrambled (Scr) siRNA. (c) Luciferase activity in HEK293T cells transfected with USP18-specific siRNA or scrambled (Scr) siRNA, and then transfected with Flag-MyD88 and Myc-USP18 or EV together with an NF-κB luciferase reporter. (d) THP-1 cells were transfected with scrambled siRNA or USP18-specific siRNA, and pre-treated with LPS for 12 hrs, followed by the treatment of LPS for the indicated time points. LPS-induced IKK, IKBα and MAPK (JNK and p38) activation were measured by immunoblotting with the indicated antibodies. (e) Quantitative comparison of signaling activation between USP18 knockdown and control cells by density scanning of the blots in (d). (f) THP-1-derived macrophage cells were transfected with Scr siRNA or USP18 siRNA (30 pmol/well) for 48 hrs. The cells were treated with LPS (100 ng/ml) for 2 hrs. The total mRNA was harvested, and IL-1β, IL-6, TNF-α mRNA abundance were analyzed by q-PCR. (g) USP18 was knocked down in THP-1 cells and THP-1-derived macrophages. IL-6 and TNF-α production was measured by ELISA after LPS treatment. Data in b, c, f, g are presented as the means ± SD of three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 versus cells transfected with scrambled siRNA (Student’s t-test).