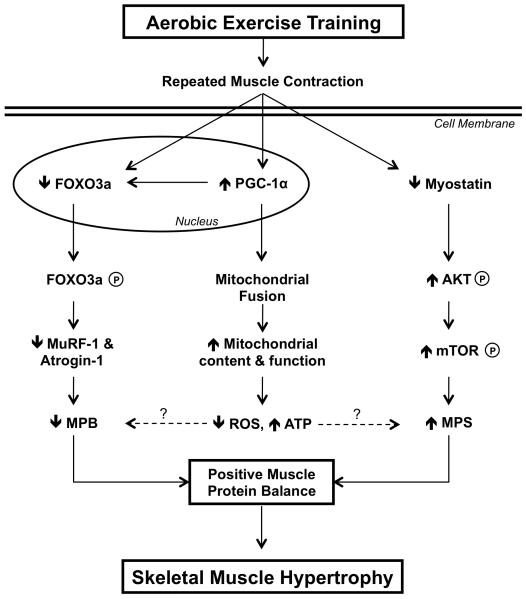
Figure 3.
In a condensed model, aerobic exercise training alters key intracellular signaling pathways and mRNA expression related to both skeletal muscle protein metabolism (e.g., FOXO3a, myostatin) (21) and mitochondrial dynamics and proliferation (22) that may be associated with skeletal muscle hypertrophy. Reduced myostatin, a negative regulator of growth, appears to promote a positive protein balance through the AKT-mTOR pathway and increased muscle protein synthesis (MPS). When phosphorylated, FOXO3a is excluded from the nucleus, which inhibits the transcription of ubiquitin E3 ligases, MuRF-1 and Atrogin-1. Reduction of MuRF-1 and Atrogin-1 may assist in lowering muscle protein breakdown (MPB) and promote a positive net protein balance. Emerging evidence (2) proposes that PGC-1α inhibits FOXO3a expression therefore lowering muscle catabolism and increasing mitochondrial biogenesis as observed after aerobic exercise training. Collectively, increased mitochondrial dynamics and abundance may lead to improved mitochondrial energetics (i.e., reduced ROS, increased ATP), which have been hypothesized to modulate MPB and MPS. Aerobic exercise appears to alter MPB and MPS to create a positive muscle protein balance and skeletal muscle growth; however, further research is needed to fully elucidate the mechanisms associated with these hypotheses. ROS = reactive oxygen species, ATP = adenosine triphosphate, P = phosphorylation.