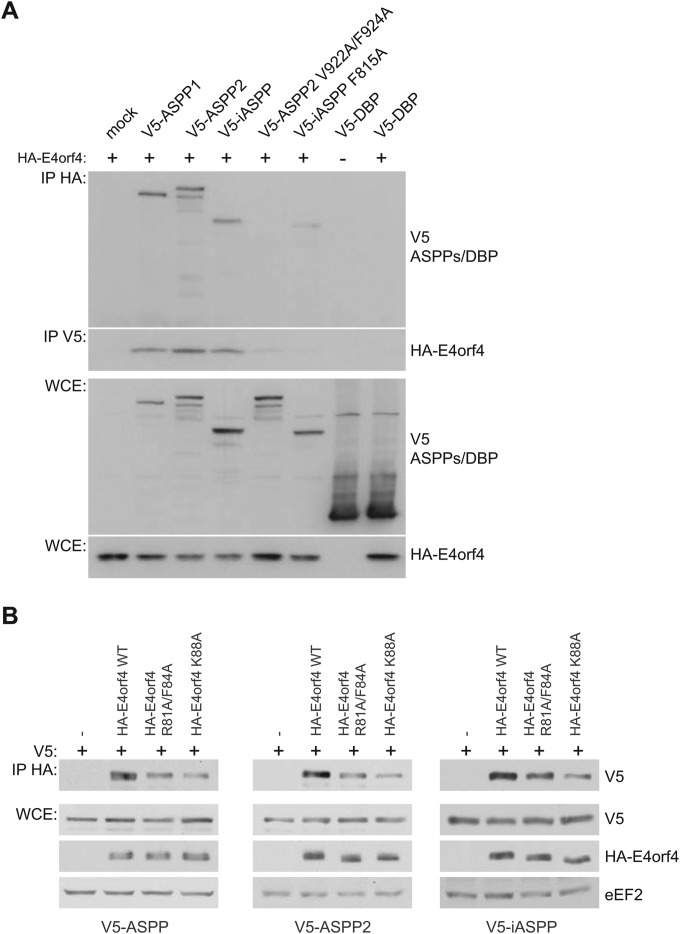
FIG 4.
E4orf4 interacts with all members of the ASPP family of proteins in a PP1 catalytic subunit-dependent manner. (A) HA-E4orf4 was immunoprecipitated using anti-HA antibodies, and Western blotting was performed using anti-V5 antibodies to detect binding of wild-type V5-ASPP subunits (ASPP1, ASPP2, and iASPP), V5-ASPP2 subunit mutated in the RVxF motif that no longer associates with PP1 catalytic subunits (ASPP2 V922A/F924A) (48), V5-iASPP subunit mutated in the RNYF motif that no longer associates with PP1 catalytic subunits (iASPP F815A) (48), or V5-DBP (adenovirus DNA binding protein) as a negative control. Reciprocal coimmunoprecipitation experiments were performed in a similar fashion. Whole-cell extracts (WCE) are shown for V5-ASPP/DBP species and HA-E4orf4. (B) H1299 cells were transfected with the indicated plasmids, and HA-E4orf4 was immunoprecipitated using anti-HA antibodies. Western blotting was performed using anti-V5 antibodies to detect binding of wild-type V5-ASPP subunits (ASPP1, ASPP2, and iASPP). Whole-cell extracts (WCE) are shown for V5-ASPP species and HA-E4orf4 and the loading control eEF2.