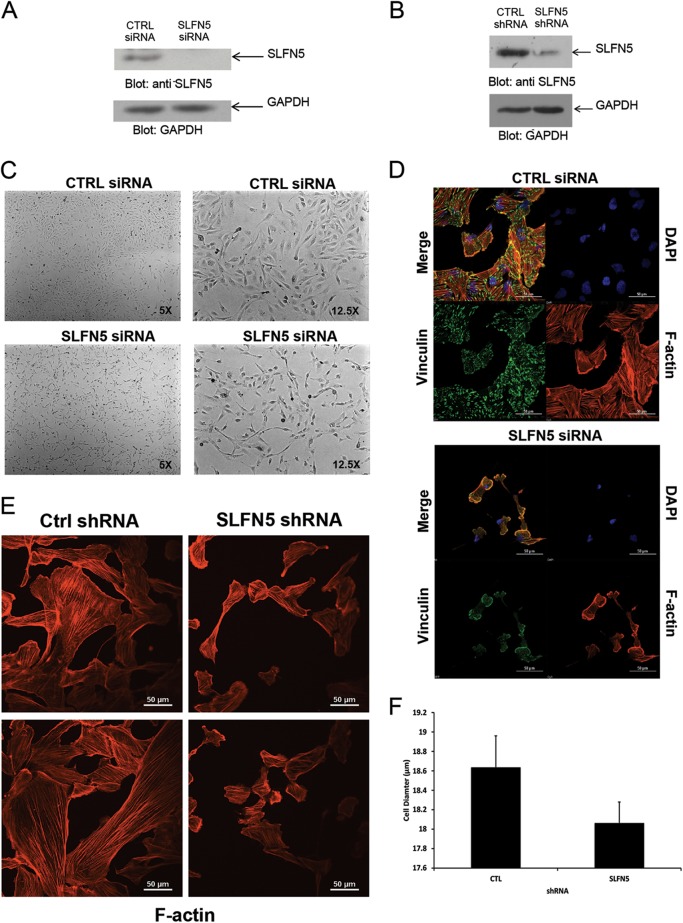
FIG 3.
Effects of SLFN5 knockdown on RCC morphology. (A) 786-0 cells were transiently transfected with control siRNA or SLFN5 siRNA, and after cell lysis, total lysates were resolved by SDS-PAGE and immunoblotted with an anti-SLFN5 antibody or an anti-GAPDH antibody, as indicated. (B) 786-0 cells stably expressing control shRNA-GFP+ or SLFN5 shRNA-GFP+ were lysed, and total cell lysates were resolved by SDS-PAGE and immunoblotted with anti-SLFN5 or anti-GAPDH antibodies, as indicated. (C) 786-0 cells were transiently transfected with control (CTRL) siRNA or SLFN5 siRNA. Forty-eight hours later, cells were imaged by phase-contrast microscopy at low (left panels) and high (right panels) magnifications. (D) 786-0 cells seeded onto glass coverslips were transiently transfected with control siRNA or SLFN5 siRNA with Lipofectamine RNAiMAX; at 48 h after transfection, the cells were fixed, permeabilized, and stained. Confocal fluorescence microscopy of focal adhesion and actin cytoskeleton was performed with triple labeling, using tetramethyl rhodamine-isothiocyanate (TRITC)-conjugated phalloidin (lower right panels), antivinculin monoclonal antibody (lower left panels), and 4′,6-diamidino-2-phenylindole (DAPI) (upper right panels). Overlay of fluorescent signals is shown in upper left panels. Images are representative areas of the entire coverslip analyzed. Bars, 50 μm. (E) 786-0 cells stably expressing control shRNA-GFP or SLFN5 shRNA-GFP were seeded for 24 h onto glass coverslips. Cells were then fixed, permeabilized, and stained with Alexa Fluor 568-labeled phalloidin to detect cytoskeletal F-actin by confocal fluorescence microscopy. Representative areas showing the F-actin structure are shown. (F) Diameter distributions of 786-0 control shRNA-GFP+ and SLFN5 shRNA-GFP+ cells were measured using the Scepter 2.0 cell counter. Differences in cell diameter are plotted on a bar graph and represent means ± standard errors from three independent counts.