FIG 11.
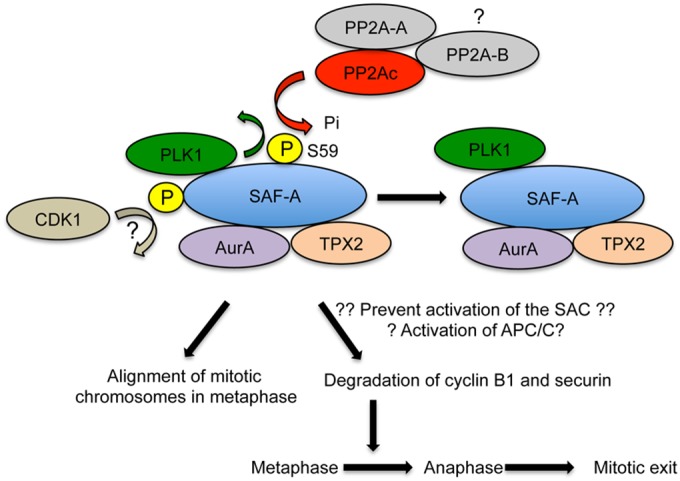
Model describing SAF-A S59 phosphorylation in mitosis SAF-A interacts with PLK1, and SAF-A S59 is phosphorylated in a PLK1-dependent manner in nocodazole-treated mitotic cells. SAF-A S59 phosphorylation also requires CDK1, possibly by phosphorylation of SAF-A on an unidentified PLK1 priming site (indicated by a question mark); however, S59 was not required for interaction of SAF-A with PLK1, Aurora A, or TPX2. SAF-A S59 is dephosphorylated by PP2A for cells to proceed from metaphase into anaphase and subsequently exit mitosis. The PP2A catalytic subunit interacts with a scaffolding A subunit and one of multiple B subunits. The PP2A-B subunit required for SAF-A S59 dephosphorylation in mitosis in not known. Inability to phosphorylate SAF-A S59 blocks degradation of cyclin B1 and securin, suggesting that SAF-A S59 phosphorylation is required for activation of the E3 ubiquitin ligase APC/C/E2 complex required for cyclin B1 and securin degradation. Alternatively, inability to phosphorylate SAF-A S59 may prevent activation of the SAC, causing misaligned mitotic chromosomes and polylobed nuclei in mitosis.
