FIG 1.
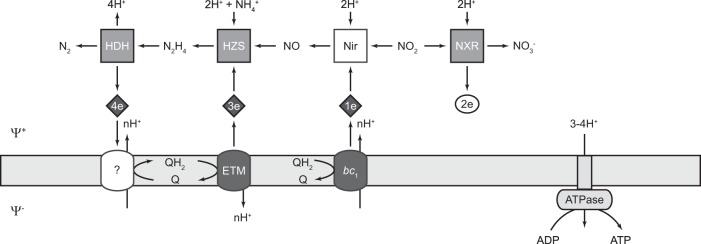
Schematic overview of anammox catabolism. Dinitrogen gas (N2) is formed from ammonium (NH4+) and nitrite (NO2−), with hydrazine (N2H4) and nitric oxide (NO) as intermediates. Diamonds represent c-type hemes involved in electron transfer between protein complexes. The number indicates the number of electrons transferred. Membrane-bound complexes are suggested to contribute to the proton motive force that is used by ATP synthase to generate ATP. Gray boxes indicate the soluble protein complexes whose intracellular locations were investigated in this study. HDH, hydrazine dehydrogenase (kustc0694); HZS, hydrazine synthase (kuste2859-60); NXR, nitrite oxidoreductase (kustd1700/03/04). NO-producing hydroxylamine oxidase (HOX; kustc1061) and the hydroxylamine oxidoreductase-like protein kustc0458 with its redox partner kustc0457, of unknown function, are not included in the scheme. The anammoxosome membrane contains the quinone (Q) pool and is energized. ψ+, positive side (anammoxosome); ψ−, negative side (cytoplasm). Other abbreviations: bc1, putative bc1 complex; ETM, electron transfer module; ATPase, F1F0-type ATP synthase; Nir, nitrite reductase. Adapted from reference 30.
