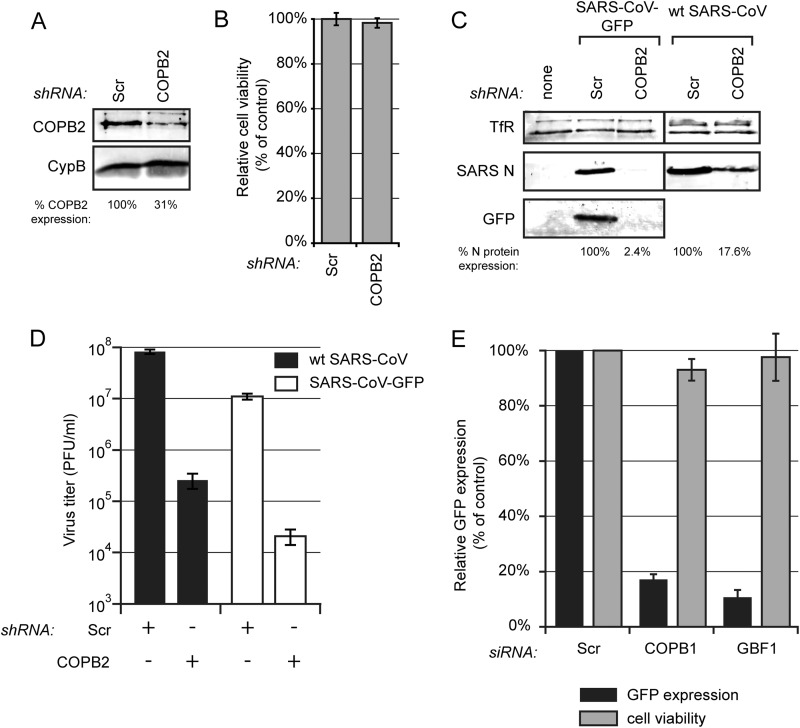
FIG 9.
Proteins of the early secretory pathway are important for SARS-CoV replication. (A) 293/ACE2 cells were transduced with lentiviruses expressing a COPB2 mRNA-specific or a scrambled shRNA. Knockdown of COPB2 expression at 48 h p.t. was monitored by Western blotting with a COPB2-specific antiserum, and cyclophilin B (CypB) was used as a loading control. (B) Viability of COPB2-depleted 293/ACE2 cells was analyzed at 48 h after transduction (percentage of control cells transduced with lentiviruses expressing a scrambled shRNA). (C and D) COPB2-depleted and control cells were infected with either SARS-CoV-GFP or wt SARS-CoV (MOI of 0.01). (C) SARS-CoV protein expression at 32 h p.i. (SARS-CoV-GFP) or 24 h p.i. (wt SARS-CoV) was analyzed by Western blotting with N-specific and GFP-specific antisera, using the TfR protein as a loading control. SARS-CoV N protein expression was quantified and normalized to that in scrambled siRNA-transfected cells (100%) as indicated under each lane. (D) SARS-CoV-GFP (white bars) and wt SARS-CoV (black bars) progeny titers in the culture supernatants of control or COPB2-depleted cells at 32 h p.i. (SARS-CoV-GFP) or 24 h p.i. (wt SARS-CoV). (E) Normalized GFP expression by SARS-CoV-GFP in 293/ACE2 cells transfected with siRNA SMARTpools targeting COPB1, GBF1, or a scrambled control siRNA. Cells were infected 48 h p.t. at an MOI of 10, and 24 h later GFP fluorescence was quantified and normalized to that in infected cells transfected with a scrambled siRNA. GFP fluorescence data are the averages from three independent experiments.