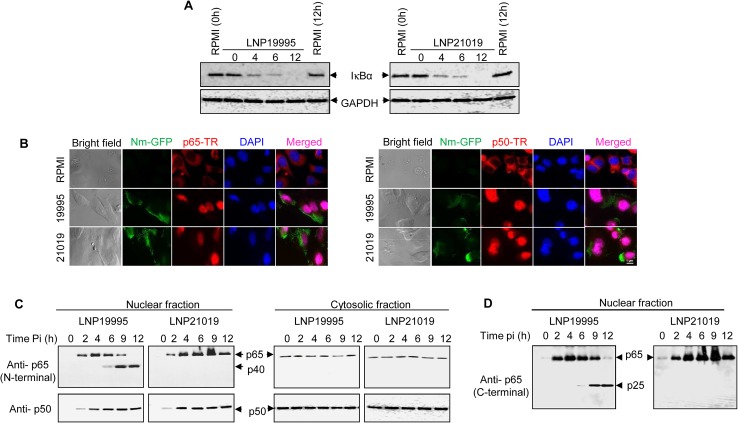
Fig 1. Meningococcal ST-11 isolates promote nuclear cleavage of p65 at late steps of infection.
(A) Meningococcal infection is accompanied by IkBα degradation. Hec-1-B epithelial cells were infected with LNP19995 (ST-11) or LNP21019 (non-ST-11) isolates for the indicated time points or left uninfected. Cytosolic fractions were subjected to immunoblotting analysis for I-κBα. Immunoblotting with anti-GAPDH antibodies was used as a protein loading control. (B) Nuclear translocation of both p65/RelA and p50/NF-κB1 subunits was not altered by meningococcal infection. Hec-1B cells were infected with GFP-expressing LNP19995 or LNP21019 or left non-infected (RPMI). After 9h, cells were fixed with 3.7% PFA, permeabilised and probed with mouse anti-p65/RelA mAb (left panel) or rabbit anti-p50/NF-κB1 (right panel) and Texas red (TR)-conjugated appropriate secondary antibody. Nuclei were stained with DAPI. Fluorescence was analyzed using immuno-fluorescence microscopy. Scale bar (1 μm) is shown. Data are representative of three independent experiments. (C) Nuclear or cytosolic fractions from (A) were analysed by immunoblotting using anti-N-terminal p65/RelA specific mAb (upper panels) or anti-p50/NF-κB1 rabbit polyclonal antibody (lower panels). Shown is a representative blot of three independent experiments. p65/RelA cleavage product p40 is indicated by arrows. (D) The blot of nuclear fractions from (C) was stripped and probed against a goat polyclonal antibody directed against C-terminal region of p65/RelA. The cleavage product p25 is indicated by arrowhead.