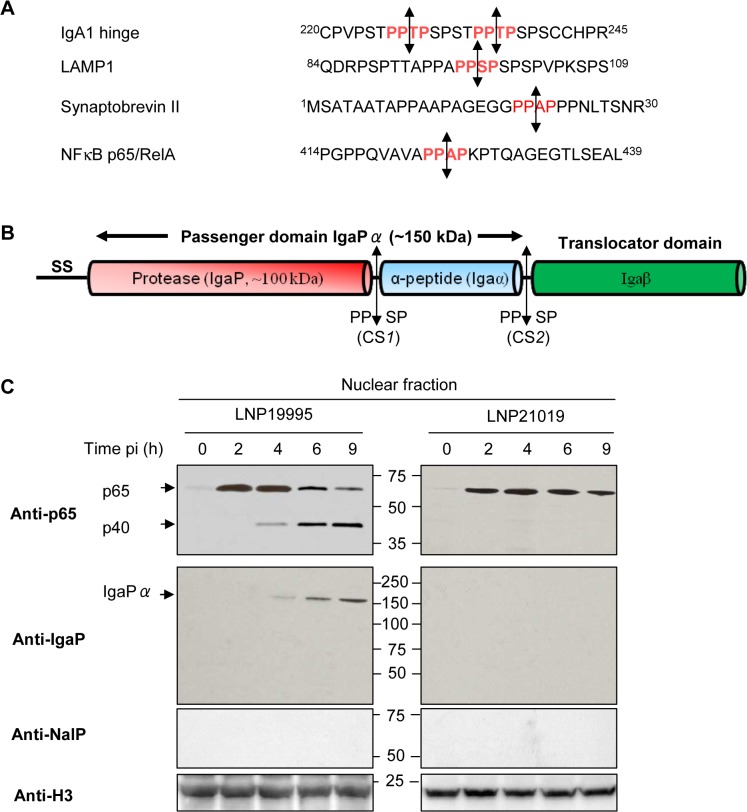
Fig 3. Cleavage of p65/RelA correlates with nuclear translocation of IgA protease.
(A) Similarity between amino acid sequences of the hinge region of human IgA1, part of the N-terminal peptide of the human lysosome-associated membrane glycoprotein 1 LAMP1 (Accession N°: AAH06345), synaptobrevin II (Accession N°: AAF15551) targeted by neisseria IgA protease and part of the carboxy terminal peptide of the p65/RelA subunit of NF-κB (Accession N°: CAA80524). The position of amino acid residues are indicated by superscript numbers delimiting each peptide sequence. Specific cleavage sites are indicated in red and the cleavage position is represented by double-headed arrows. (B) Schematic overview of the various domains and sub-domains of neisserial IgA protease. Autocatalyic cleavage sites CS1 and CS2 and their sequences are indicated by double-headed arrows. SS: signal sequence. (C) Nuclear fractions were prepared from Hec-1-B cells infected with LNP19995 or LNP21019 at indicated time points. Samples were resolved in SDS-PAGE and probed with anti-N-terminal p65 mAb or rabbit polyclonal serum specific to IgaP sub-domain or anti-NalP specific serum. Histone H3 was used as loading control.