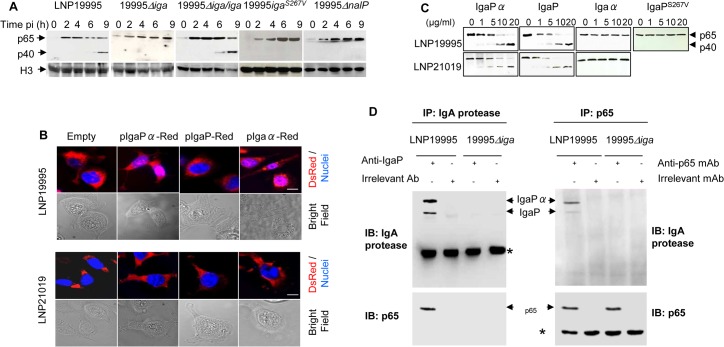
Fig 4. IgA protease of ST-11 isolates interacts with and mediates nuclear cleavage of p65/RelA.
(A) Hec-1-B cells were infected with the indicated strains. For each time point, nuclear fractions were prepared and analysed by immunoblotting using anti-p65 mAb. Histone H3 was used as loading control. (B) Localization of IgA protease sub-domains in transfected infected cells. IgaP, Igaα or IgaPα of LNP19995 (upper panel) or LNP21019 (lower panel) were fused to DsRed. Hec-1-B cells were then transfected with either construct or pDsRedN1 plasmid (Empty vector). After 48 hours, cells were washed, fixed with 3.7% PFA and stained with DAPI (blue) before visualisation under the microscope. Scale bar (10 μm) is shown. (C) In vitro activity of purified IgA protease passenger sub-domains against p65. IgaP, Igaα or IgaPα or IgaPS267V of the strains LNP19995 (upper panel) and LNP21019 (lower panel) were subcloned and purified as C-terminal His6-tagged proteins. Nuclear extracts (1 μg) from LPS-stimulated Hec-1-B cells were mixed with the indicated concentrations of the purified proteins. The reaction mixtures were incubated at 25°C for 3 hours and then analyzed with antibodies against p65/RelA (N-terminal specific). (D) IgA protease interacts with p65/RelA. Nuclear extracts from LPS-stimulated Hec-1-B cells were mixed with MSP prepared from LNP19995 or 19995Δiga for 6h in presence of 5 mM PMSF. The samples were immunoprecipitated with anti-IgaP specific serum or irrelevant rabbit Ab (left panel) or anti-p65/RelA mAb or irrelevant mAb (right panel) and analyzed by immunoblotting with antibodies against p65/RelA and IgaP, respectively. * indicates antibody heavy chain.