Abstract
To quantitatively assess disease burden due to tuberculosis between populations residing in and outside of urban informal settlements in Rio de Janeiro, Brazil, we compared disability-adjusted life years (DALYs), or “DALY-gap.” Using the 2010 Brazilian census definition of informal settlements as aglomerados subnormais (AGSN), we allocated tuberculosis (TB) DALYs to AGSN vs non-AGSN census tracts based on geocoded addresses of TB cases reported to the Brazilian Information System for Notifiable Diseases in 2005 and 2010. DALYs were calculated based on the 2010 Global Burden of Disease methodology. DALY-gap was calculated as the difference between age-adjusted DALYs/100,000 population between AGSN and non-AGSN. Total TB DALY in Rio in 2010 was 16,731 (266 DALYs/100,000). DALYs were higher in AGSN census tracts (306 vs 236 DALYs/100,000), yielding a DALY-gap of 70 DALYs/100,000. Attributable DALY fraction for living in an AGSN was 25.4 %. DALY-gap was highest for males 40–59 years of age (501 DALYs/100,000) and in census tracts with <60 % electricity (12,327 DALYs/100,000). DALY-gap comparison revealed spatial and quantitative differences in TB burden between slum vs non-slum census tracts that were not apparent using traditional measures of incidence and mortality. This metric could be applied to compare TB burden or burden for other diseases in mega-cities with large informal settlements for more targeted resource allocation and evaluation of intervention programs.
Keywords: Disease burden, DALY, Disability-adjusted life years, DALY-gap, Urban informal settlements, Slums, Tuberculosis
Introduction
More than one billion people worldwide live in urban informal settlements, defined by the United Nations Human Settlements Program (UN-Habitat) as slums.1 This population represents 43 % of the combined urban populations in all developing countries.1 Residence in slums is a risk factor for a variety of adverse health outcomes. Slum dwellers share a greater burden of such health outcomes than their non-slum community counterparts residing in the same city.2,3 However, the magnitude of such difference in disease burden between these communities is not evident by routine surveillance reporting incidence or mortality. The age of onset of a disease, as well as the duration of disability associated with that disease, cannot be directly quantified by incidence alone. As such, there are no studies that have quantitatively compared disease burden in urban slum vs non-slum residents within one entire city. One measure of disease burden—disability-adjusted life years (DALYs) reported in the 2010 Global Burden of Disease Survey (GBD-2010)—aggregated measures across regions and nations.4 The same method, however, can be applied to compare disease burden among subpopulations within nations, such as in urban slum vs non-slum dwellers. Slum dwellers are likely to live longer with disability resulting from medical conditions (years lived with disability or YLD) and more likely to suffer more years of life lost (YLL) than non-slum residents in the same city when they develop the same disease or injuries. Such disease burden measurements would allow quantitative evaluation of the effectiveness of intervention strategies and implementation of targeted health policies to mitigate health disparities among different subpopulations in a nation.
As the population of urban informal settlements continues to expand in mega-cities of the world, targeted urban health intervention strategies become urgently needed. In the present study, we applied the 2010 GBD metrics to compare tuberculosis (TB) burden in urban slum vs non-slum communities in Rio de Janeiro, Brazil. TB transmission is associated with several characteristics that define informal settlements, including overcrowding, poor housing quality, lack of health education and services, and inadequate implementation of TB contact tracing programs.5–8 These conditions engender other adverse health outcomes unrelated to TB. Despite significant allocation of resources to combat TB with a rigorous national TB control program,9 the city of Rio de Janeiro reported the second highest TB incidence rates in the country in 2010.10 In addition, Rio de Janeiro has Brazil’s largest population of slum dwellers, as defined by the Brazilian National 2010 Census Report.11 TB remains a leading cause globally of DALYs among young adult males aged 15–39 years of age,4 the largest age group in the slums of Rio de Janeiro.12 Here, we quantitatively compared TB DALYs between formal and informal neighborhoods of Rio de Janeiro to assess differences in the burden of this disease. We propose a new metric called “DALY-gap” as a way to quantitate disease burden between two communities in the same city. Benefits and barriers to scaling up this metric for other diseases and regions are discussed.
Methods
Study Population and Census Tract Designation
We analyzed data from the Brazilian Information System for Notifiable Diseases (Sistema de Informação de Agravos de Notificação—SINAN) for all confirmed, incident tuberculosis (TB) cases reported in 2005 and 2010 that occurred in the municipality of Rio de Janeiro. SINAN is the national notifiable disease surveillance system of Brazil developed in the 1990s to unify and disseminate information for improved disease monitoring and control. With respect to TB, SINAN includes patient socio-demographic characteristics and TB infection characteristics, risk factors, treatment, and outcome. Quality assessments of this database have been performed routinely since its establishment, making it a recognized reliable source of disease data.9 SINAN works in three levels. First, the case is reported to the municipality health department where the data are entered into the SINAN database. This data are then sent to the state health department, such that cases transferred between municipalities can be tracked and duplicates removed. Then, the information is merged into a national database and deduplicated. Data aggregated by municipality/state/country is published publicly online (http://dtr2004.saude.gov.br/sinanweb/index.php). TB is a mandatory notifiable disease in Brazil. All patients, whether they use the public or private healthcare system, need to have their suspected diagnosis reported to the municipality health department (as part of the Brazilian Unified Health System, Sistema Único de Saúde—SUS) in order to receive treatment for TB. This authorization is required to receive or purchase TB medications.
In the 2010 census survey, the Brazilian Census Bureau operationally defined informal settlements as aglomerados subnormais (AGSN) or subnormal settlements. According to this definition, AGSNs are census tracts with at least 51 housing units on illegally occupied land with construction outside of existing municipal patterns or non-secure access to essential public services.11 Many of the components of the AGSN definition are similar to those of the UN-Habitat’s definition of slums.1 Geographic shape files and census data were obtained from open-access database of the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística—IBGE).13 In 2010, the IBGE defined AGSN border limits. As the 2000 census did not distinguish AGSN vs non-AGSN populations, the population estimates from the 2010 census tracts were used for both 2005 and 2010 calculations. The population of Rio de Janeiro in 2010 was 6,299,684 inhabitants, with 1,391,953 inhabitants living within AGSN census tracts.13
All cases were geocoded by address and mapped in ArcGIS 10 (ESRI, Redlands, CA). Case residence was intersected with AGSN census tract limits plus a 50-m buffer. The buffer zone was incorporated to account for two AGSN residential address characteristics: (1) residents frequently register their address as a collective mailbox located immediately outside AGSN limits and (2) cases that map to the street precision level are mapped to the last available street number closest to the AGSN limits. Therefore, cases mapped to within a 50-m radius of an AGSN census track boundary were considered as living in the AGSN census tract. Cases that could not be mapped to the address or street precision were excluded. Incarcerated and homeless cases were not assigned to a census tract and hence were not included in the analysis comparing AGSN and non-AGSN tracts.
DALY Calculation
DALYs were calculated as the sum of years of life lost (YLLs) and incident years lived with disability (YLDs) based on the 2010 Global Burden of Disease study methodology described elsewhere.4 Given the high quality of Brazilian TB case reporting to the SINAN database, a prospective method or incidence approach for calculating DALYs was employed. Consistent with the 2010 GBD methodology, age-weighting and discounting were not applied. Incident YLDs were calculated by TB incidence multiplied by estimated disease duration and disability weights for TB sequelae.14 Using data from the state of Rio de Janeiro SINAN database, we calculated disease durations by sex and HIV status for each group as weighted averages considering total disease durations of 9, 12, and 24 months for cases that ended in death, cure, and loss to follow-up.15 As the average duration of disease did not differ significantly between HIV positive and negative cases nor between males and females, a standard duration of 1.1 years was applied for all incident TB cases. We calculated YLLs by subtracting age of death from TB from the reference life expectancy at that age.16 TB mortality was considered as “death by tuberculosis” registered in SINAN in 2010 by the health clinic responsible for the case. YLLs were not calculated for 2005 because TB was not listed as a specific cause of death in SINAN prior to 2007. Given this limitation, DALYs were calculated for 2010 only.
DALY-gap
DALY-gap was calculated as the difference in age-adjusted DALYs per 100,000 population attributable to cases residing in AGSN census tracts from those residing in non-AGSN census tracts. Of 10,504 census tracts of Rio de Janeiro, 309 were uninhabited and not included in the analysis. Census tract DALYs were stratified by AGSN and non-AGSN and categorized by percentage of census tract residents with access to adequate electricity, sanitation, solid waste disposal, and water services. We also compared mean monthly income based on increments of the Brazilian national monthly minimum wage in 2010 (US$290), average number of residents per household, and percentage of inhabitants who rent their living space. DALY density was calculated as DALYs per population divided by the total square kilometer area for AGSN and non-AGSN census tracts. Data analysis was performed in SAS 9.3 (SAS Institute. Inc., Cary, NC). DALYs per population were mapped by census tract, with AGSN/non-AGSN census tracts designated with different colors. Maps highlighted areas from the three major metropolitan zones of Rio de Janeiro.
Ethical Approval
This study was approved by the Ethics Committee of the Federal University of Espírito Santo, which has also obtained approval from the Brazilian Ministry of Health to access the SINAN database.
Results
TB incidence in Rio de Janeiro decreased from 120/100,000 person-years (7534 cases) in 2005 to 116/100,000 person-years (7276 cases) in 2010. Excluded for census tract designation were 301 (142 in 2010) cases mapped outside of the municipality’s limits or with unidentified addresses. Of the total cases, 755 (452 in 2010) were incarcerated and 140 (81 in 2010) were identified as homeless. In 2010, TB incidence per population in AGSN census tracts (n = 1807, 130/100,000 person-years) was higher than in non-AGSN census tracts (n = 4794, 98/100,000 person-years) (Table 1).
TABLE 1.
Tuberculosis years lived with disability (YLDs), years of life lost (YLLs), and disability-adjusted life years (DALYs) stratified by cases residing in informal settlement (aglomerados subnormais—AGSN) and non-AGSN census tracts of Rio de Janeiro in 2010
| Census tract | Age | Number | n/100,000 population | YLD (%) |
YLD/100,000 population | YLL (%) |
YLL/100,000 population | DALY (%) |
DALY/100,000 population |
|---|---|---|---|---|---|---|---|---|---|
| Non-AGSN | <20 | 425 | 35.7 | 156.2 (8.7) | 13.1 | 642.8 (6.6) | 53.9 | 798.6 (6.9) | 67.0 |
| 20–39 | 2125 | 138.3 | 797.5 (44.6) | 51.9 | 4154.7 (42.4) | 270.4 | 4952.2 (42.7) | 322.3 | |
| 40–59 | 1634 | 122.0 | 611.9 (34.2) | 45.7 | 3840.0 (39.2) | 286.8 | 4452.0 (38.4) | 332.5 | |
| >60 | 609 | 72.6 | 223.4 (12.5) | 26.6 | 1170.3 (11.9) | 139.6 | 1393.7 (12.0) | 166.2 | |
| Total | 4793 | 97.7 | 1789.0 (100.0) | 36.5 | 9807.8 (100.0) | 199.9 | 11,596.4 (100.0) | 236.4 | |
| AGSN | <20 | 235 | 47.3 | 86.8 (12.9) | 17.5 | 350.6 (9.7) | 70.5 | 437.0 (10.2) | 87.9 |
| 20–39 | 864 | 172.4 | 322.4 (47.9) | 64.3 | 1372.1 (38.1) | 273.8 | 1694.4 (39.7) | 338.1 | |
| 40–59 | 565 | 192.0 | 211.1 (31.4) | 71.8 | 1515.4 (42.1) | 515.1 | 1726.5 (40.4) | 586.8 | |
| >60 | 143 | 141.2 | 53.0 (7.9) | 52.3 | 359.6 (10.0) | 355.0 | 412.5 (9.7) | 407.3 | |
| Total | 1807 | 129.7 | 673.2 (100.0) | 48.3 | 3597.6 (100.0) | 258.1 | 4270.4 (100.0) | 306.4 | |
| DALY-Gap | 32.0 | 11.8 | 58.2 | 70.0 |
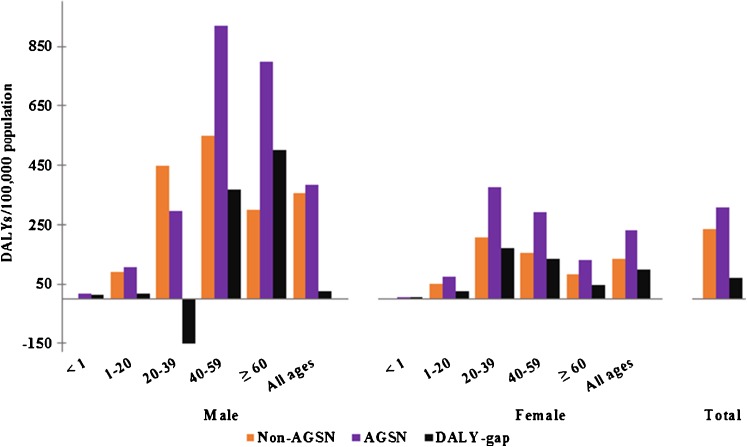
Total DALYs/100,000 population in 2010 for all TB cases, including incarcerated and homeless, were 265.6. DALYs resulting from HIV positive cases contributed 36.1 % of total DALYs, while those from incarcerated and homeless populations accounted for 4.1 % of total DALYs. DALYs were higher in AGSN census tracts than non-AGSN census tracts (306.4 vs 236.4 DALYs/100,000 pop), resulting in a DALY-gap of 70.0 DALYs/100,000 pop (Table 1). Attributable DALY fraction for living in an AGSN that contributed to total TB DALYs for 2010 was 25.4 %. DALY-gap was highest in males 40–59 years (500.6 DALYs/100,000 pop) and in females 20–39 years (169.4 DALYs/100,000 pop) (Fig. 1). A negative DALY-gap (−149.4/100,000) was observed only in males aged 20–39 years, with higher DALYs per population occurring in non-AGSN census tracts for males of this age group.
FIG. 1.
Age-specific disability-adjusted life years (DALYs)/100,000 population for male and female tuberculosis cases in 2010. DALY-gap per 100,000 population is denoted for each age group.
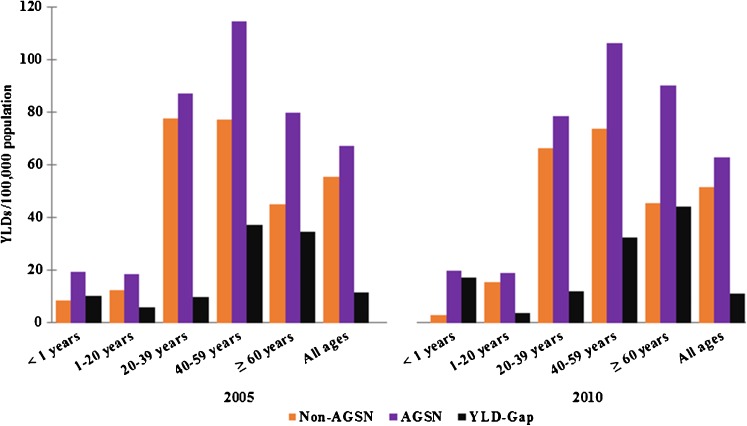
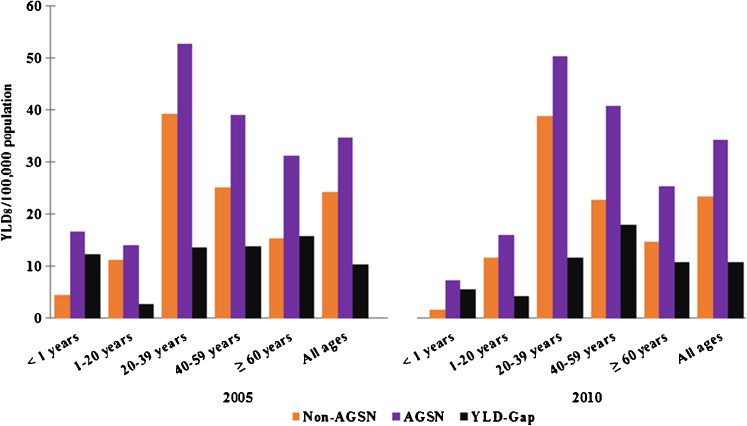
Total TB YLDs/100,000 population decreased from 41.3 in 2005 to 39.1 in 2010. During this same period, YLD-gap between AGSN and non-AGSN census tracts experienced a minimal decrease, but increased specifically in infants less than 1 year and in those 60 years or older (+0.1 and +1.1 YLDs/100,000, respectively). Figures 2 and 3 compare the distribution of YLDs across age groups for males and females, respectively, in 2005 and 2010. YLD patterns remained consistent over this 5-year period among both males and females, with the exception that the highest TB burden in males shifted from 45 to 64 years in 2005 to 65 years and older in 2010. YLD-gap increased from 2005 to 2010 for females. Additionally, YLD-gap in male infants nearly doubled over the time period.
FIG. 2.
Age-specific years lived with disability (YLDs)/100,000 population for male tuberculosis cases in 2005 and 2010. YLD-gap per 100,000 population is denoted for each age group.
FIG. 3.
Age-specific years lived with disability (YLDs)/100,000 population for female tuberculosis cases in 2005 and 2010. YLD-gap per 100,000 population is denoted for each age group. Note: scale is different from Fig. 2.
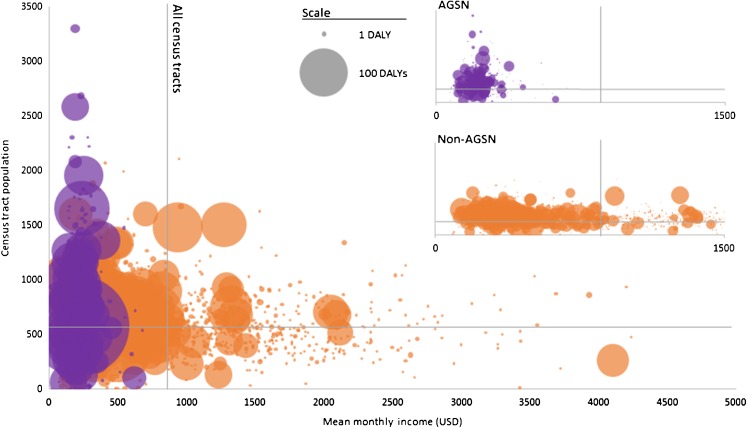
Highest DALYs per population were found in AGSN census tracts with the poorest housing and sanitation conditions, while estimates were more evenly distributed among non-AGSN census tracts with varying rates of public service connectivity. The highest TB burden per population was found in AGSN census tracts with <60 % electricity, sanitation, and water accessibility (12,385.3; 12,385.3; and 467.0 DALYs/100,000 population, respectively) and <70 % solid waste disposal coverage (635.6 DALYs/100,000 population) (Table 2). TB burden in non-AGSN census tracts was notably higher in census tracts with mean monthly income less than the 2010 minimum wage. Regardless of AGSN or non-AGSN census tract, the large majority of DALYs occurred in census tracts below the average 2010 monthly household income for Rio de Janeiro (Fig. 4). AGSN and non-AGSN combined DALY-gap between the lowest and highest income census tracts was 323.1 DALYs/100,000 population (505.7 DALYs/100,000 for non-AGSN census tracts only). DALY-gap by income could not be calculated for AGSN census tracts because there was no population registered in the highest income bracket.
TABLE 2.
Tuberculosis disability-adjusted life years (DALYs) by informal settlement (aglomerados subnormais—AGSN) and non-AGSN census tracts of Rio de Janeiro in 2010
| Census tract characteristic | Total | AGSN | Non-AGSN | |||
|---|---|---|---|---|---|---|
| DALY | DALY/100,000 population | DALY | DALY/100,000 population | DALY | DALY/100,000 population | |
| Mean monthly income in $USD | ||||||
| <290 | 1222 | 437 | 741 | 367 | 481 | 619 |
| 290–579 | 6496 | 293 | 3255 | 290 | 3240 | 296 |
| 580–1448 | 6353 | 262 | 249 | 378 | 6104 | 259 |
| 1449–5793 | 1257 | 96 | 0 | 0 | 1257 | 96 |
| ≥5794 | 57 | 114 | 0 | – | 57 | 114 |
| Average persons per household | ||||||
| 1–2 | 7320 | 237 | 961 | 381 | 6358 | 224 |
| 3–4 | 8062 | 254 | 3282 | 291 | 4779 | 233 |
| 5 or more | 3 | 21 | 1 | 12 | 1 | 89 |
| % Rented households | ||||||
| <10 % | 3111 | 312 | 1665 | 392 | 1447 | 253 |
| 10–19 % | 4359 | 199 | 1019 | 235 | 3340 | 190 |
| 20–29 % | 3736 | 199 | 547 | 221 | 3189 | 196 |
| ≥30 % | 4172 | 343 | 1014 | 355 | 3158 | 339 |
| % Households with electricity | ||||||
| <60 % | 69 | 1100 | 66 | 12,385 | 3 | 58 |
| 60–69 % | 73 | 788 | 0 | – | 73 | 788 |
| 70–79 % | 178 | 2015 | 0 | – | 178 | 2015 |
| 80–89 % | 196 | 424 | 0 | 0 | 196 | 437 |
| ≥90 % | 14,863 | 239 | 4180 | 301 | 10,683 | 222 |
| % Households with sanitation | ||||||
| <60 % | 71 | 1080 | 66 | 12,385 | 5 | 88 |
| 60–69 % | 71 | 724 | 0 | 0 | 71 | 791 |
| 70–79 % | 180 | 1797 | 0 | – | 180 | 1797 |
| 80–89 % | 199 | 408 | 0 | 24 | 198 | 420 |
| ≥90 % | 14,858 | 239 | 4180 | 301 | 10,678 | 222 |
| % Households with solid waste disposal | ||||||
| <60 % | 245 | 598 | 172 | 576 | 72 | 659 |
| 60–69 % | 145 | 714 | 71 | 847 | 74 | 621 |
| 70–79 % | 201 | 663 | 22 | 165 | 179 | 1043 |
| 80–89 % | 308 | 305 | 104 | 311 | 204 | 302 |
| ≥90 % | 14,480 | 238 | 3877 | 297 | 10,603 | 222 |
| % Households with water | ||||||
| <60 % | 225 | 276 | 206 | 467 | 19 | 51 |
| 60–69 % | 78 | 257 | 2 | 23 | 75 | 367 |
| 70–79 % | 221 | 461 | 5 | 42 | 217 | 592 |
| 80–89 % | 395 | 346 | 70 | 259 | 325 | 373 |
| ≥90 % | 14,460 | 241 | 3962 | 305 | 10,497 | 223 |
En dash indicates categories in which there was no population
FIG. 4.
Absolute disability-adjusted life years (DALYs) per census tract by mean monthly household income ($US) and census tract population in 2010. Purple denotes informal settlement (aglomerados subnormais—AGSN) census tracts and orange non-AGSN census tracts. Circle size represents absolute DALYs of census tracts. Individual graphs for AGSN and non-AGSN census tracts were provided in the upper right-hand corner (reduced scale). Vertical and horizontal gray lines represent mean monthly income and total population, respectively, for all Rio de Janeiro census tracts (with and without TB cases).
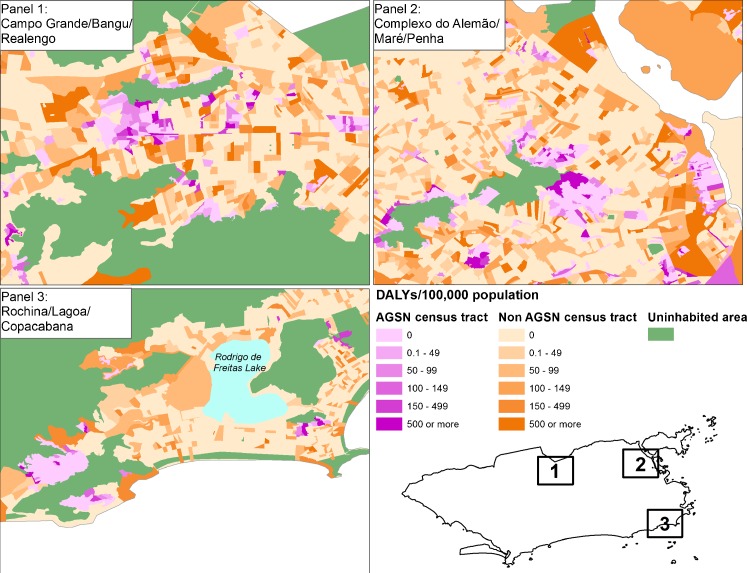
DALY density was over five times greater in AGSN census tracts (77.3 DALYs/km2 for AGSN and 14.3 DALYs/km2 for non-AGSN). DALY maps which highlight areas from three main Zones of Rio de Janeiro revealed distinct geographical DALY patterns among census tracts (Fig. 5). AGSN census tracts represented concentrated areas of high TB DALY burden. Non-AGSN census tracts with high TB burden mainly bordered AGSN census tracts and uninhabited areas and were located in low-income areas.
FIG. 5.
Disability-adjusted life years (DALYs)/100,000 population by census tract in three highlighted areas of Rio de Janeiro, Brazil in 2010. Purple denotes AGSN census tracts and orange non-AGSN census tracts.
Discussion
By calculating DALY-gap to assess TB burden in Rio de Janeiro, several key spatial and demographic characteristics of TB burden between the slum and non-slum communities became evident that were not apparent by other traditional measures of TB incidence and mortality. We applied this metric to examine TB in Rio de Janeiro for the following reasons: (1) case reporting of TB is uniform and comprehensive in the city, (2) the city has the second highest concentration of slum dwellers in Brazil, (3) it reports the second highest number of TB cases in the country.10,11 Even though the overall TB incidence decreased between 2005 and 2010, the YLD-gap between slum and non-slum communities increased during this time, indicating the persistent or worsening structural factors that contribute to greater TB burden in Rio’s informal communities.
The comparison of differences in TB burden in two types of communities in the same city by measuring DALY-gap unmasked neighborhood characteristics that would have been missed by traditional measures of incidence. For example, in a well-known tourist neighborhood of Copacabana, there is an AGSN census tract community Morro do Cantagalo, which has an average monthly income of US$423 and a population of 1652 residents. This community is situated about 1.5 km from one of the highest income census tracts of Copacabana, which has an average monthly income of US$2189 and a population of 237 residents. Interestingly, the high-income neighborhood actually reported a higher incidence of TB in 2010 than Morro do Cantagalo (22 vs. 15 cases, or 9283/100,000 vs. 908/100,000 person-years). Yet, the burden of TB in Morro do Cantagalo was considerably higher (132 DALYs) than its high-income counterpart (8 DALYs). Per population, Morro do Cantagalo suffered over double the number of DALYs/100,000 population (7990 vs. 3443), an astounding DALY-gap of 4547 DALYs/100,000. Incidence alone would suggest TB control programs should target the high-income neighborhood over the slum community; however, DALY-gap revealed the opposite in disease burden between the two communities.
TB DALYs per population based on the GBD-2010 report was 142 DALYs/100,000 for Brazil.4,13 In the present study, TB DALYs per population in Rio was 266 DALYs/100,000 population, demonstrating the higher disease burden incurred in the urban mega-city. Furthermore, the TB DALY-gap between slum vs non-slum census tracts in Rio was 70 DALYs/100,000 population. Our findings are consistent with a recent study in Rio de Janeiro on healthy life expectancy (HALE), another measure of morbidity and mortality based on disability. Adults living in slum census tracts (AGSNs) were found to have approximately half the HALE than those residing in wealthy census tracts across all age groups.17 Taken together, the studies provide quantitative evidence that slum residents suffer disproportionately the weight of ill-health that occur in the same city.
Another study conducted in three slums in Nairobi, Kenya, calculated the YLLs specific to informal settlements.18 The authors found AIDS and TB combined were the leading contributors to YLLs in those older than 5 years, accounting for nearly 50 % of the mortality burden in these informal settlements. However, the study did not directly compare the YLLs of these slums with those for the region or with non-slum communities. Without comparable values for formal communities, it is not possible to determine the fraction of YLL burden attributable to living in a slum. Much of the previous work using DALYs to identify health disparities within specific regions has focused on ethnic disparities.19–22 However, DALY-gap in slum communities is different from income or indigenous health gap; it addresses disease burden of communities or neighborhoods, making it a metric easier to apply for resource allocation. Furthermore, the spatial analysis in our study showed that the TB burden differs greatly even across different slum and non-slum communities.
We found over one fourth of all TB DALYs in Rio could be attributed to living in a slum. Incarcerated and homeless cases, two populations often associated with high TB infection rates, also contributed to TB disease burden (2.7 and 1.5 % of DALYs, respectively). However, as there are no population estimates for these groups in Rio de Janeiro, their TB burden could not be compared to other census tract populations. Interestingly, among males aged 20–39 years, DALY/population was higher among residents of non-AGSN (Fig. 1). DALY-gap for males of this age group was −149 DALYs/100,000. We found that 63.1 % of DALYs (82.1 % of cases) of the incarcerated cases with TB were in males of the same age group. The residential addresses of the incarcerated people were not available. It is conceivable that a large proportion of these incarcerated people may be residents of AGSNs and hence the TB DALY in this age group may be underestimated in AGSN in our analysis.
Of particular concern is the DALY-gap in infants less than 1 year of age, especially in male infants in which YLD-gap nearly doubled from 2005 to 2010. TB infection in the infant population suggests ongoing transmission and can be used as an indicator of an inadequate contact tracing program.23 While the overall TB burden in infants decreased over time, which would indicate a successful intervention program, DALY-gap revealed this reduction did not take place in the slum population. This disparity suggests TB control programs in Rio may not be reaching these at-risk populations and provides an opportunity to identify interventions that will be effective in reducing disease burden in this population.
Highest TB burden was found in census tracts with mean monthly income lower than the minimum wage, regardless of whether or not they were indicated as slum or non-slum. Disease- and injury-specific studies based on the GBD-2010 data have shown national per-capita income to be a strong predictor of mortality and DALY loss rates.19,24 Here, the two lowest income groups contributed 94.1 % to the total burden of TB among slum residents and 33.4 % among residents of non-slum census tracts. Thus, low income is an important contributor for TB DALY in the slum population, but factors other than low income are also important contributors to TB burden among the non-slum population.
While DALYs measured across nations or regions have shortcomings, several limitations also exist for scaling up the DALY-gap metric for other diseases and subpopulations. Our study was made possible by the comprehensive census survey conducted in 2010 by Brazil that divided census tracts according to a formal definition and mapping of informal settlements as AGSN, and that TB reporting is uniform and high in Brazil. One limitation, however, is that it is not possible to distinctly demarcate neighborhoods into formal and informal settlements in large cities like Rio de Janeiro. The burden of TB in non-AGSN census tracts was highest in those tracts that bordered AGSN census tracts. These bordering zones actually have many of the characteristics that define AGSNs. Thus, the DALY-gap we describe here is likely to be underestimated.
The Brazilian SINAN is a well-validated surveillance system that includes TB as a major targeted disease. Case-detection rate of SINAN reporting range was estimated to be 88 % in 2010.9 Without these census and survey data, DALY-gap measurements would not have been possible to calculate. While countries like India and Kenya have made efforts to conduct census and define geographic boundaries of their urban informal settlements,25,26 other countries with mega-cities have yet to do so. Without definition of slum boundaries, DALY-gap will remain difficult to assess accurately.
Another limitation of comparing DALYs across subpopulations is that the same disability weight and disease duration for incident YLDs is applied for all cases.16 Furthermore, slum populations were likely underrepresented in the most recent survey on disability weights for GBD 2010 and concordance of disability weights across different cultures and demographics may have been overstated.27 It is very likely that residents of slum communities experience a longer duration of disability when they develop a disease than residents of non-slum communities who develop the same disease.2,3 Those who develop symptoms of TB in an informal settlement are more likely to take a longer time to seek medical care than non-slum residents who develop TB, resulting in a more advanced disease at diagnosis. If such individuals develop multidrug-resistant (MDR)TB, they will have a longer duration of disability, as MDRTB will require a longer duration of treatment. As the same TB disease duration was used to calculate YLD for all cases in the present study, DALY-gaps observed here were conservative estimates. Future DALY-gap studies using subpopulation-adapted disability weights, prevalence, and/or different slum vs non-slum disease durations will likely reveal larger differences.
DALY is already a widely used, validated methodology accessible to all governments and researchers across the globe. The expansion of DALY-gap methodology to other regions, subpopulations, diseases, and risk factors may promote better understanding of the burden of disease attributable to living in these underserved communities. Temporal measures of DALY-gap will be an invaluable metric to assess the effectiveness of slum upgrading projects, such as those being implemented in preparation for Brazil’s major international sporting events, such as the World Cup in 2014 and the Olympics in 2016.
Acknowledgement
Funding
MM was supported by NIH Research Training Grant #R25TW009338 (Global Health Equity Scholars Program) funded by the Fogarty International Center and the Office of AIDS Research at the National Institutes of Health. ELNM, CMMS, and TG were supported by Grant #U2RTW006885 ICOHRTA from the Secretariat of Health Surveillance/Ministry of Health of Brazil.
References
- 1.UN-HABITAT. The challenge of slums: global report on human settlements 2003. London and Sterling. London, UK: Earthscan Publications Ltd; 2003.
- 2.Riley LW, Ko AI, Unger A, Reis MG. Slum health: diseases of neglected populations. BMC International Health and Human Rights. 2007; 7(2). doi: 10.1186/1472-698x-7-2 [DOI] [PMC free article] [PubMed]
- 3.Unger A, Riley LW. Slum health: from understanding to action. PLoS Med. 2007;4(10):1561–6. doi: 10.1371/journal.pmed.0040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 5.Maciel ELN. Health promotion and social determinants of tuberculosis: elements for action. In: Landim FLP, Catrib AMF, Collares PMC, editors. Health promotion in human diversity and plurality of therapeutic itineraries. São Paulo, Brazil: Saberes Editora; 2012. pp. 429–49. [Google Scholar]
- 6.Harling G, Castro MC. A spatial analysis of social and economic determinants of tuberculosis in Brazil. Health Place. 2014;25:56–67. doi: 10.1016/j.healthplace.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Hossain S, Quaiyum MA, Zaman K, et al. Socio economic position in TB prevalence and access to services: results from a population prevalence survey and a facility-based survey in Bangladesh. PLoS ONE. 2012;7(9):e44980. doi: 10.1371/journal.pone.0044980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lienhardt C. From exposure to disease: the role of environmental factors in susceptibility to and development of tuberculosis. Epidemiol Rev. 2001;23(2):288–301. doi: 10.1093/oxfordjournals.epirev.a000807. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira GP, Torrens AW, Bartholomay P, et al. Tuberculosis in Brazil: last ten years analysis – 2001–2010. Braz J Infect Dis. 2013;17(2):218–33. doi: 10.1016/j.bjid.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ministério da Saúde, Brasil. Tuberculosis incidence rate. 2010. http://tabnet.datasus.gov.br/cgi/tabcgi.exe?idb2011/d0202.def. Accessed January 9, 2014.
- 11.Instituto Brasileiro de Geografia e Estatística. 2010 Demographic census—subnormal settlements territorial information, 2011. ftp://ftp.ibge.gov.br/Censos/Censo_Demografico_2010/Aglomerados_subnormais/Aglomerados_subnormais_informacoes_territoriais/aglomerados_subnormais_informacoes_territoriais.pdf. Accessed November 1, 2013.
- 12.Snyder RE, Jaimes G, Riley LW, et al. A comparison of social and spatial determinants of health between formal and informal settlements in a large metropolitan setting in Brazil. J Urban Health: Bull N Y Acad Med. 2014;91(3):432–445. doi: 10.1007/s11524-013-9848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Instituto Brasileiro de Geografia e Estatística. 2010 Census. 2010. http://censo2010.ibge.gov.br/. Accessed January 9, 2014.
- 14.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129–43. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leite IC, Valente JG, Schramm JMA, et al. National and regional estimates of disability-adjusted life-years (DALYs) in Brazil, 2008: a systematic analysis. Lancet. 2013;381:S83. doi: 10.1016/S0140-6736(13)61337-9. [DOI] [Google Scholar]
- 16.Murray CJ, Ezzati M, Flaxman AD, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380(9859):2063–6. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 17.Szwarcwald CL, da Mota JC, Damacena GN, et al. Health inequalities in Rio de Janeiro, Brazil: lower healthy life expectancy in socioeconomically disadvantaged areas. Am J Public Health. 2011;101(3):517–23. doi: 10.2105/AJPH.2010.195453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyobutungi C, Ziraba AK, Ezeh A, et al. The burden of disease profile of residents of Nairobi’s slums: results from a demographic surveillance system. Popul Health Metrics. 2008;6:1. doi: 10.1186/1478-7954-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polinder S, Haagsma JA, Toet H, et al. Burden of injury in childhood and adolescence in 8 European countries. BMC Public Health. 2010;10:45. doi: 10.1186/1471-2458-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aragon TJ, Lichtensztajn DY, Katcher BS, et al. Calculating expected years of life lost for assessing local ethnic disparities in causes of premature death. BMC Public Health. 2008;8:116. doi: 10.1186/1471-2458-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costilla R, Tobias M, Blakely T. The burden of cancer in New Zealand: a comparison of incidence and DALY metrics and its relevance for ethnic disparities. Aust N Z J Public Health. 2013;37(3):218–25. doi: 10.1111/1753-6405.12062. [DOI] [PubMed] [Google Scholar]
- 22.Vos T, Barker B, Begg S, et al. Burden of disease and injury in Aboriginal and Torres Strait Islander Peoples: the Indigenous health gap. Int J Epidemiol. 2009;38(2):470–7. doi: 10.1093/ije/dyn240. [DOI] [PubMed] [Google Scholar]
- 23.Adhikari M. Tuberculosis and tuberculosis/HIV co-infection in pregnancy. Semin Fetal Neonatal Med. 2009;14(4):234–40. doi: 10.1016/j.siny.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol. 2009;8(4):345–54. doi: 10.1016/S1474-4422(09)70023-7. [DOI] [PubMed] [Google Scholar]
- 25.Government of India. Report of the committee on slum statistics/census, Ministry of Housing and Urban Poverty Alleviation, New Delhi. 2010. http://mhupa.gov.in/W_new/Slum_Report_NBO.pdf. Accessed May 5, 2014.
- 26.Emina J, Beguy D, Zulu EM, et al. Monitoring of health and demographic outcomes in poor urban settlements: evidence from the Nairobi urban health and demographic surveillance system. J Urban Health: Bull N Y Acad Med. 2011;88(Suppl 2):200–218. doi: 10.1007/s11524-011-9594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nord E. Disability weights in the Global Burden of Disease 2010: unclear meaning and overstatement of international agreement. Health Policy. 2013;111(1):99–104. doi: 10.1016/j.healthpol.2013.03.019. [DOI] [PubMed] [Google Scholar]