Figure 2.
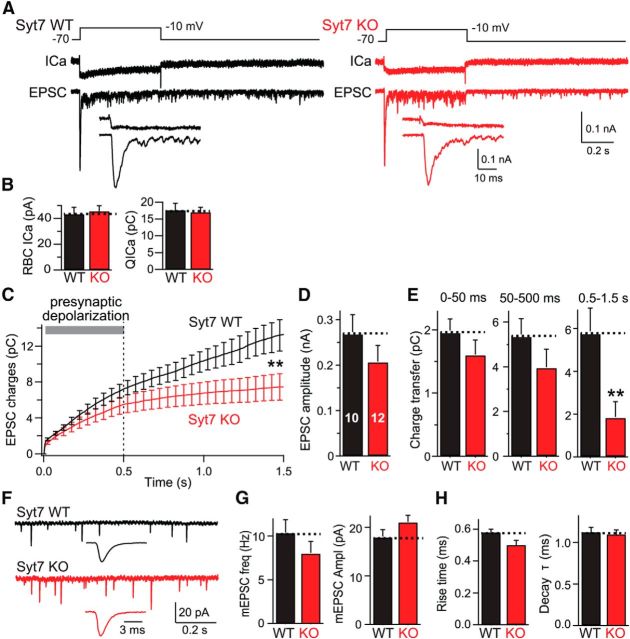
Syt7 KO selectively impairs delayed asynchronous release at RBC–AII cell synapses. A, Experimental design (top), representative presynaptic Ca2+ current traces (ICa; middle), and postsynaptic EPSC traces (bottom) recorded from connected RBCs and AII cells in littermate WT (left) and Syt7 KO (right) mice. The insets show the initial 50 ms of the EPSC at an expanded timescale. Note that these measurements were performed under standard conditions using an internal pipette solution with 1.3 mm BAPTA. B, Summary graph of the Ca2+ current amplitude (left) and integrated Ca2+ charge transfer (right) shows that the Syt7 KO has no effect on Ca2+ influx. C, Plot of the cumulative EPSC charge transfer during and after the 500 ms depolarization reveals selective impairment in Syt7 KO synapses of delayed asynchronous release after the end of the depolarization. D, Summary graph of peak EPSC amplitudes (corresponding to fast synchronous release) measured in WT and Syt7 KO mice. E, Summary graphs of the charge transfer integrated over the initial 50 ms of depolarization (fast synchronous release), the remaining depolarization period lasting from 50 to 500 ms (sustained release), and the 1 s period after the depolarization (0.5–1.5 s, delayed asynchronous release). Only delayed asynchronous release is significantly different between WT and KO mice. F–H, Analysis of spontaneous mEPSCs recorded in AII cells from WT and Syt7 KO mice (F, representative traces; G, summary graphs of mEPSC frequency and amplitude; H, summary graphs of mEPSC rise and decay times). All data are means ± SEMs (n = 10–12). Statistical analyses were performed by Student's t test comparing Syt7 KO with WT littermates, except an ANOVA followed by the post hoc Bonferroni's test was used in C and E. **p < 0.01.