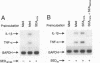Abstract
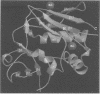
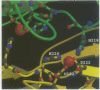
Bacterial superantigens, including the staphylococcal enterotoxins, are the most potent activators of T cells known and have been suggested as a causative factor in Gram-positive shock in humans. Staphylococcal enterotoxin D (SED) is dependent upon Zn2+ for high affinity interactions with MHC class II molecules and thus SED was co-crystallized with Zn2+. The crystal structure of SED has been determined in two different space groups, at 2.3 and 3.0 A resolution respectively. The three-dimensional structure of SED is similar to structures of other bacterial superantigens, although this study has revealed that SED has the unique capability of forming dimers in the presence of Zn2+. The high affinity Zn2+ site used in dimer formation is located on the surface of the beta-sheet in the C-terminal domain. Two bound metal ions are coordinated by residues from both molecules in the dimer interface and thus contribute directly to formation of the dimer. A second Zn2+ site is located on the surface close to the domain interface of the molecule. The unique feature of SED in forming a Zn2+-dependent homodimer seems to facilitate novel and biologically relevant multimeric interactions with MHC class II molecules, as shown by the induction of cytokine mRNA in human monocytes when exposed to SED and SED mutants.
Full text
PDF

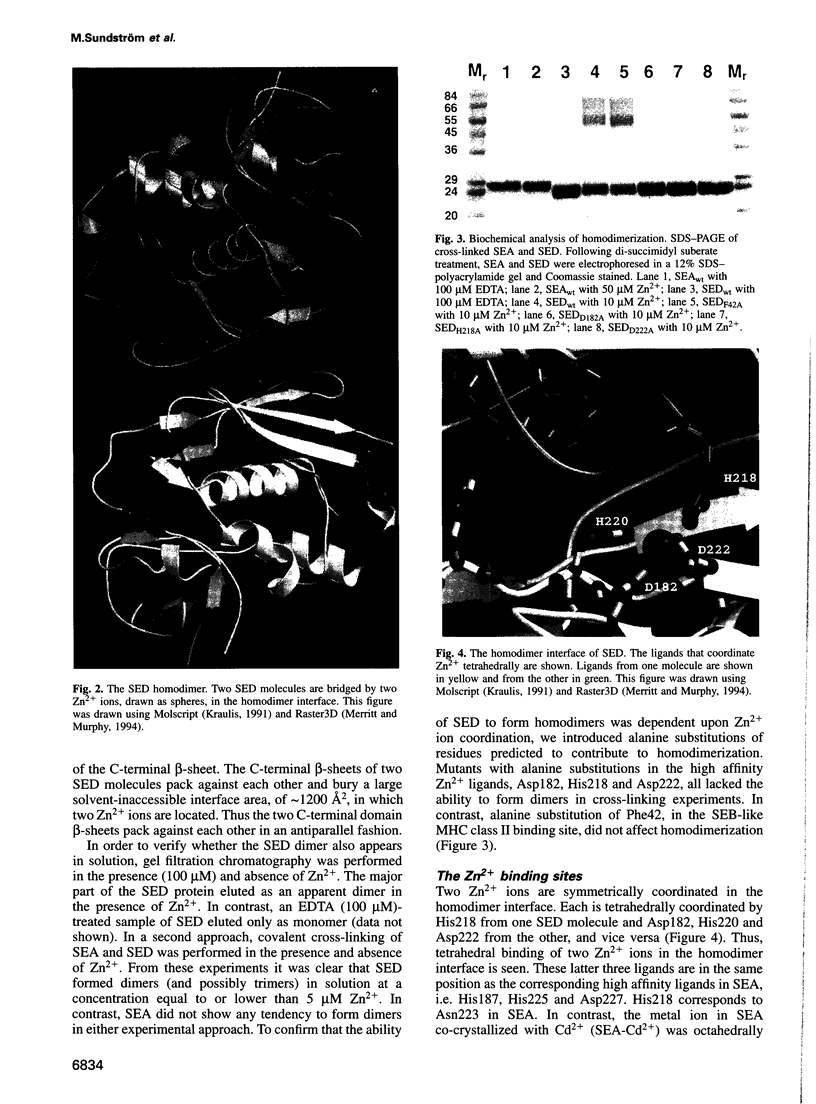
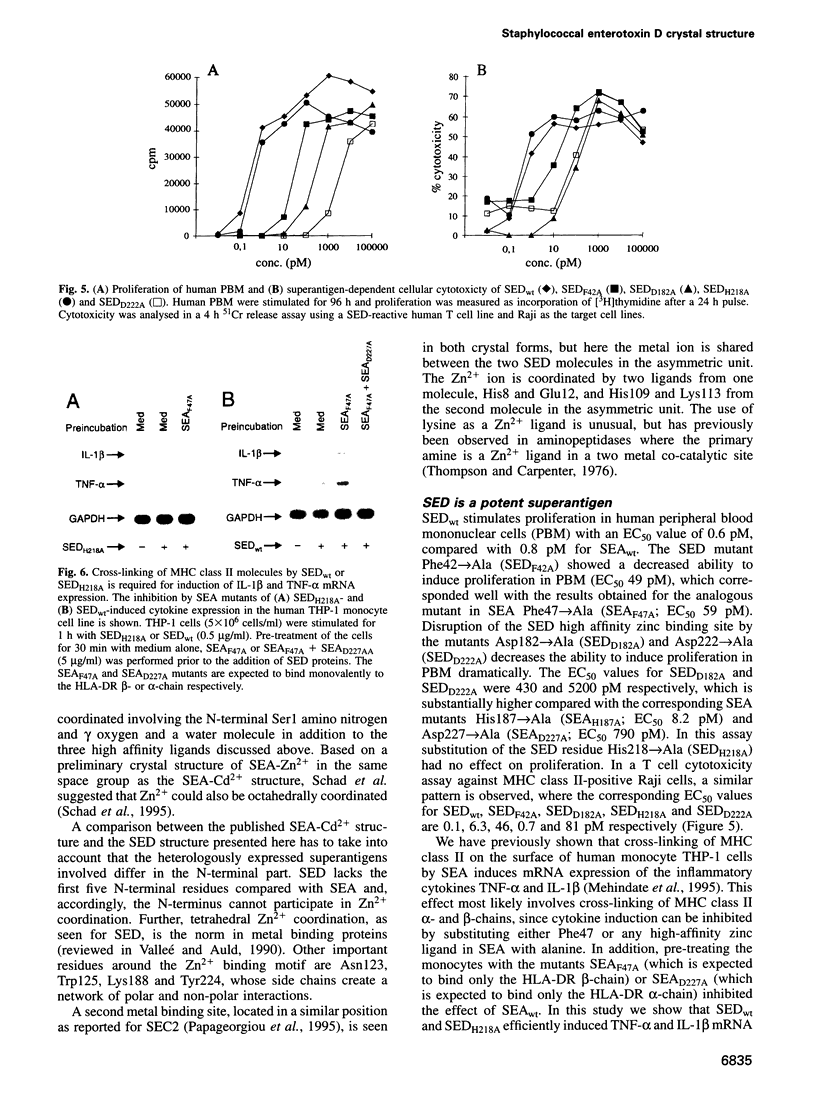
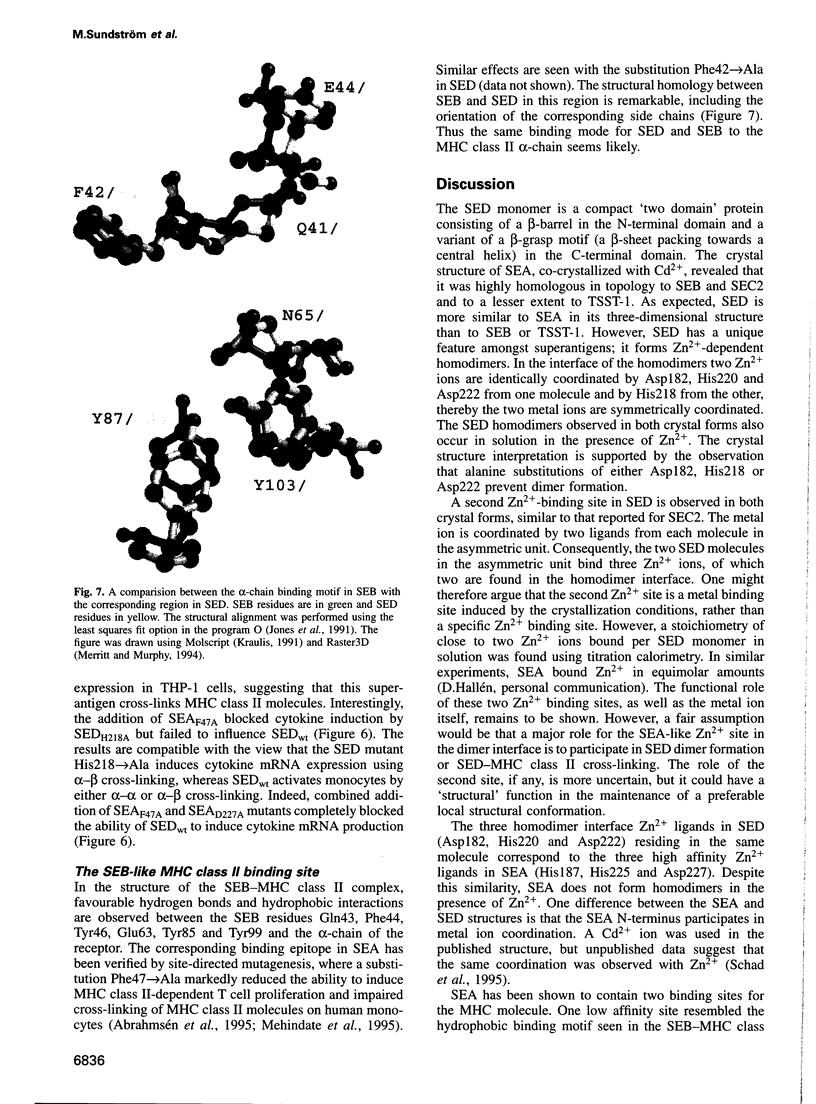



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahmsén L., Dohlsten M., Segrén S., Björk P., Jonsson E., Kalland T. Characterization of two distinct MHC class II binding sites in the superantigen staphylococcal enterotoxin A. EMBO J. 1995 Jul 3;14(13):2978–2986. doi: 10.1002/j.1460-2075.1995.tb07300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya K. R., Passalacqua E. F., Jones E. Y., Harlos K., Stuart D. I., Brehm R. D., Tranter H. S. Structural basis of superantigen action inferred from crystal structure of toxic-shock syndrome toxin-1. Nature. 1994 Jan 6;367(6458):94–97. doi: 10.1038/367094a0. [DOI] [PubMed] [Google Scholar]
- Bayles K. W., Iandolo J. J. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J Bacteriol. 1989 Sep;171(9):4799–4806. doi: 10.1128/jb.171.9.4799-4806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley M. J., Mekalanos J. J. Nucleotide sequence of the type A staphylococcal enterotoxin gene. J Bacteriol. 1988 Jan;170(1):34–41. doi: 10.1128/jb.170.1.34-41.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull J. M., Pappin D. J., Mark J., Aebersold R., Köster H. Functionalized membrane supports for covalent protein microsequence analysis. Anal Biochem. 1991 Apr;194(1):110–120. doi: 10.1016/0003-2697(91)90157-o. [DOI] [PubMed] [Google Scholar]
- Dohlsten M., Abrahmsén L., Björk P., Lando P. A., Hedlund G., Forsberg G., Brodin T., Gascoigne N. R., Förberg C., Lind P. Monoclonal antibody-superantigen fusion proteins: tumor-specific agents for T-cell-based tumor therapy. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8945–8949. doi: 10.1073/pnas.91.19.8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlsten M., Hedlund G., Akerblom E., Lando P. A., Kalland T. Monoclonal antibody-targeted superantigens: a different class of anti-tumor agents. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9287–9291. doi: 10.1073/pnas.88.20.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. D., Urban R. G., Strominger J. L., Robinson H. Zinc regulates the function of two superantigens. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5507–5511. doi: 10.1073/pnas.89.12.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A., Labrecque N., Thibodeau J., Marrack P., Kappler J. W., Sekaly R. P. Identification of the staphylococcal enterotoxin A superantigen binding site in the beta 1 domain of the human histocompatibility antigen HLA-DR. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9954–9958. doi: 10.1073/pnas.88.22.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson K. R., Tiedemann R. E., Urban R. G., Lowe S. C., Strominger J. L., Fraser J. D. Staphylococcal enterotoxin A has two cooperative binding sites on major histocompatibility complex class II. J Exp Med. 1995 Sep 1;182(3):711–720. doi: 10.1084/jem.182.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardetzky T. S., Brown J. H., Gorga J. C., Stern L. J., Urban R. G., Chi Y. I., Stauffacher C., Strominger J. L., Wiley D. C. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature. 1994 Apr 21;368(6473):711–718. doi: 10.1038/368711a0. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Karp D. R., Long E. O. Identification of HLA-DR1 beta chain residues critical for binding staphylococcal enterotoxins A and E. J Exp Med. 1992 Feb 1;175(2):415–424. doi: 10.1084/jem.175.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Urban R. G., Strominger J. L., Wiley D. C. Toxic shock syndrome toxin-1 complexed with a class II major histocompatibility molecule HLA-DR1. Science. 1994 Dec 16;266(5192):1870–1874. doi: 10.1126/science.7997880. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Mehindate K., Thibodeau J., Dohlsten M., Kalland T., Sékaly R. P., Mourad W. Cross-linking of major histocompatibility complex class II molecules by staphylococcal enterotoxin A superantigen is a requirement for inflammatory cytokine gene expression. J Exp Med. 1995 Nov 1;182(5):1573–1577. doi: 10.1084/jem.182.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt E. A., Murphy M. E. Raster3D Version 2.0. A program for photorealistic molecular graphics. Acta Crystallogr D Biol Crystallogr. 1994 Nov 1;50(Pt 6):869–873. doi: 10.1107/S0907444994006396. [DOI] [PubMed] [Google Scholar]
- Papageorgiou A. C., Acharya K. R., Shapiro R., Passalacqua E. F., Brehm R. D., Tranter H. S. Crystal structure of the superantigen enterotoxin C2 from Staphylococcus aureus reveals a zinc-binding site. Structure. 1995 Aug 15;3(8):769–779. doi: 10.1016/s0969-2126(01)00212-x. [DOI] [PubMed] [Google Scholar]
- Prasad G. S., Earhart C. A., Murray D. L., Novick R. P., Schlievert P. M., Ohlendorf D. H. Structure of toxic shock syndrome toxin 1. Biochemistry. 1993 Dec 21;32(50):13761–13766. doi: 10.1021/bi00213a001. [DOI] [PubMed] [Google Scholar]
- Schad E. M., Zaitseva I., Zaitsev V. N., Dohlsten M., Kalland T., Schlievert P. M., Ohlendorf D. H., Svensson L. A. Crystal structure of the superantigen staphylococcal enterotoxin type A. EMBO J. 1995 Jul 17;14(14):3292–3301. doi: 10.1002/j.1460-2075.1995.tb07336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström M., Hallén D., Svensson A., Schad E., Dohlsten M., Abrahmsén L. The Co-crystal structure of staphylococcal enterotoxin type A with Zn2+ at 2.7 A resolution. Implications for major histocompatibility complex class II binding. J Biol Chem. 1996 Dec 13;271(50):32212–32216. doi: 10.1074/jbc.271.50.32212. [DOI] [PubMed] [Google Scholar]
- Swaminathan S., Furey W., Pletcher J., Sax M. Crystal structure of staphylococcal enterotoxin B, a superantigen. Nature. 1992 Oct 29;359(6398):801–806. doi: 10.1038/359801a0. [DOI] [PubMed] [Google Scholar]
- Thompson G. A., Carpenter F. H. Leucine aminopeptidase (bovine lens). The relative binding of cobalt and zinc to leucine aminopeptidase and the effect of cobalt substitution on specific activity. J Biol Chem. 1976 Mar 25;251(6):1618–1624. [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990 Jun 19;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]