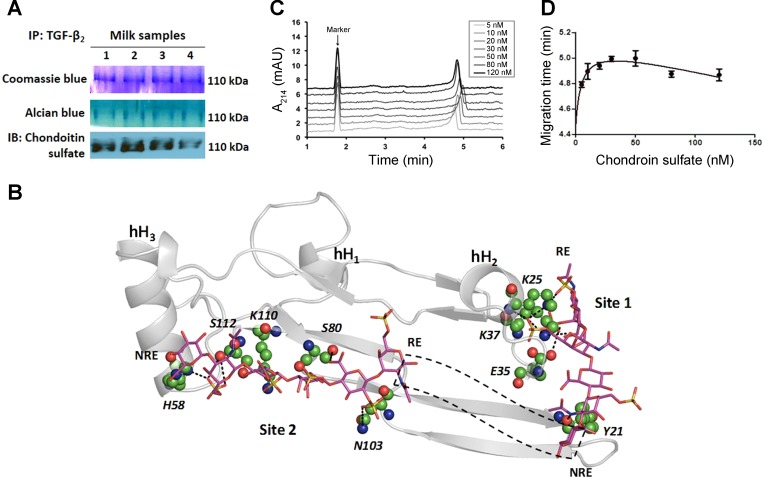
Fig. 2.
TGF-β2 is bound to chondroitin sulfate proteoglycans in milk. A: we immunoprecipitated TGF-β2 and resolved the immune complexes in nondenaturing gels. Staining with coomassie blue (top) or alcian blue (middle) dyes showed a prominent 110-kDa band. The same band also showed immunoreactivity for chondroitin sulfate. Data represent preterm milk samples from four mothers, tested in at least three separate runs. B: computational model predicts the binding of a chondroitin sulfate hexasaccharide sequence to TGF-β2 at two potential binding sites (Site 1, Site 2). Chondroitin sulfate sequence is depicted in a sticks form, whereas TGF-β2 is depicted in grey ribbons with individual residues predicted to be involved in some interaction at an atomic level in ball and stick form. Helices are denoted as hH1, hH2, and hH3. Site 1 residues include Lys25, Lys37, Glu35, and Tyr91 and Site 2 residues include His58, Ser80, Asn103, Lys110, and Ser112. Dotted black box depicts the possible orientation of intervening chondroitin sulfate residues connecting the reducing end (RE) of Site 2-bound chain to the nonreducing end (NRE) of Site 1-bound chondroitin sulfate chain. C: affinity capillary electrophoresis demonstrates biophysical interaction between chondroitin sulfate and TGF-β2. Panel shows electropherogram of 0.5 μM TGF-β2 in sodium phosphate buffer containing different concentrations of chondroitin sulfate. Dimethyl sulfoxide is the marker. Data represent findings from three separate experiments. D: binding curve of chondroitin sulfate-TGF-β2 system, fitted for a one-site total binding equation.