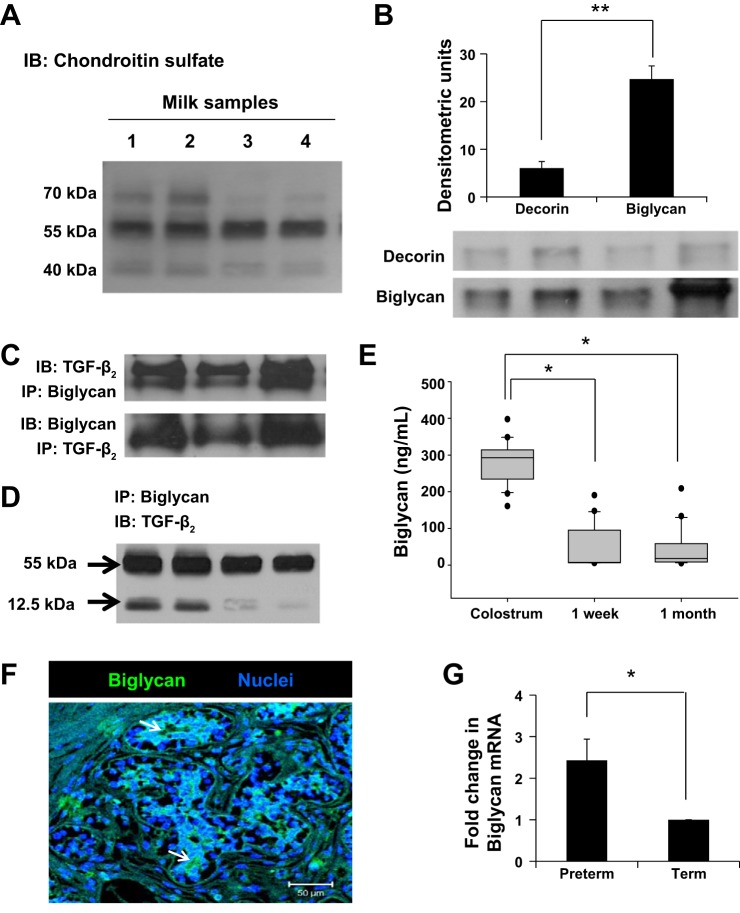
Fig. 3.
Human milk contains important amounts of biglycan. A: representative Western blots on immune complexes obtained upon immunoprecipitation of TGF-β2 from milk showed three prominent bands in the 40- to 70-kDa range. Data show milk samples from four mothers. B: representative Western blots detected chondroitin sulfate proteoglycans decorin and biglycan attached to TGF-β2 in preterm human milk. These immunoblots were performed on protein complexes obtained by immunoprecipitation of TGF-β2. Bar diagram (means ± SE) summarizes densitometric data. C: nondenaturing gels following immunoprecipitation with anti-TGF-β2 or antibiglycan antibodies confirm that biglycan was bound to TGF-β2 in milk. Data represent milk samples from three different mothers. D: representative Western blots show that biglycan binds both latent (55 kDa) and active TGF-β2 (12.5 kDa) in milk. These immunoblots were performed on protein complexes obtained by immunoprecipitation of biglycan. E: box-whisker plots show temporal change in biglycan concentrations in preterm milk. Biglycan concentrations were measured by ELISA. N = 20 mothers; each sample tested in triplicate. *P < 0.05. F: immunofluorescence photomicrograph (magnification ×250) of human mammary tissue shows biglycan immunoreactivity (green) in epithelial cells. Nuclear staining (blue) was obtained with 4′,6-diamidino-2-phenylindole (DAPI). Data represent results in tissue from three different subjects. G: biglycan mRNA expression in the cellular fraction of preterm vs. term milk, normalized against GAPDH. Bar diagram (means ± SE) shows fold changes in biglycan mRNA expression in preterm milk over term milk. N = 8 each samples; tested in triplicate. *P < 0.05, **P < 0.01.