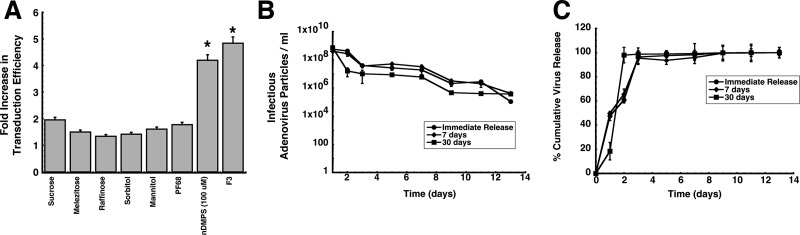
Figure 1.
Multicomponent formulations improve adenovirus transduction efficiency and stabilize virus in PLGA microspheres. (A) Transduction efficiency of excipients and formulations in differentiated Calu-3 cells. Cell monolayers were exposed to formulations containing a model recombinant adenovirus serotype 5 vector expressing beta-galactosidase (AdlacZ) for 2 h at 37 °C. Transduction efficiency was determined by comparison of the number of cells expressing the beta-galactosidase transgene after treatment with formulated virus to the number of beta-galactosidase positive cells after treatment with virus in saline. Results are reported as the mean ± standard error of the mean of data generated from triplicate samples over three separate experiments (n = 9 each formulation). PF68, Pluronic F68; nDMPS, N-dodecyl-β-d-maltopyranoside; F3, formulation containing sucrose (10 mg/mL), mannitol (40 mg/mL), and 1% (v/v) poly(ethylene) glycol 3,000. * indicates a significant difference with respect to unformulated virus. (B) Adenovirus concentration versus time profiles of supernatants collected from PLGA microspheres stored at 37 °C. Ten milligrams of microspheres containing AdlacZ was suspended in 0.5 mL of sterile saline immediately after preparation (Immediate Release) or after storage at room temperature (25 °C) for 7 or 30 days. The number of infectious particles released at each time point was determined by serial dilution of collected supernatants and subsequent infection of Calu-3 cells. (C) In vitro release profiles of adenovirus from PLGA microspheres stored at room temperature over time. Release rates for freshly prepared beads did not significantly differ from those of beads stored at 25 °C for 7 days. The release rate increased 3-fold after storage for one month under the same conditions. Results depicted in panels B and C are reported as the mean ± standard error of the mean of data generated from triplicate samples collected from six separate experiments.