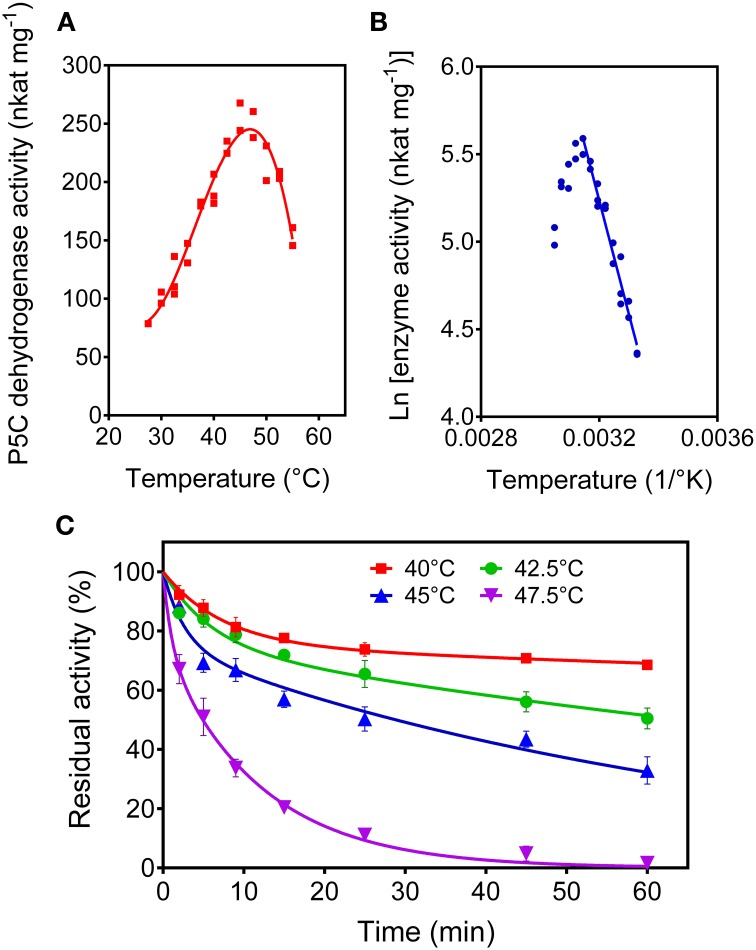
Figure 9.
Thermal stability of rice P5C dehydrogenase. The activity rate of the purified enzyme was measured for up to 5 min under standard assay conditions at increasing temperatures (A). Replotting data in the so-called Arrhenius plot (B) allowed the calculation of the activation energy (Table 2). Thermal stability of the enzyme was determined by incubating aliquots for increasing time at increasing temperature in the absence of substrates (C). After the indicated times, the aliquots were immediately re-equilibrated on ice and the residual activity was then measured at 35°C, and expressed as percentage of activity in untreated controls. Three replicates were carried out for each treatment, and means ± SE over replicates are shown.