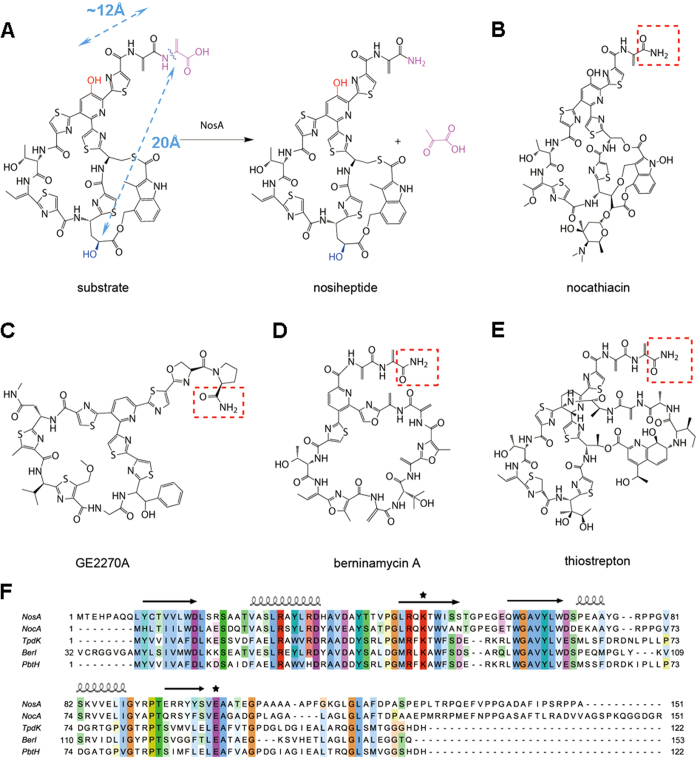
Figure 1. NosA and its homologs share a common mechanism on the post-modifications of thiopeptides.
(A) The catalytic reaction for nosiheptide maturation by NosA. The –OH groups involved in the interactions with NosA are highlighted in red and blue, respectively. The Cα-N bond cleavage site is marked in pink and a wavy line. The distances between the oxygen atoms of the –OH groups and the Cα-N bond cleavage site in the substrate were measured as 12 Å and 20 Å, indicated by blue dotted lines, respectively. (B–D) the C-terminal amide formation of the thiopeptides similar to nosiheptide, such as nocathiacin (catalyzed by NocA), (C) GE2270A (catalyzed by TpdK or PbtH). (D) berninamycin A (catalyzed by BerI). (E) For comparison, thiostrepton maturation is done through deesterification and amidation by TsrB and TsrC, respectively. In (B–E), the products of the catalytic reactions were highlighted in red dashed boxes. (F) The sequence alignments of NosA and its homologs NocA, TpdK, PbtH and BerI. The conserved residues K49 and E101 were marked with stars on the top of the sequences. On the top of the amino acid sequence, the secondary structures of NosA were displayed, where arrows indicate β-sheets, and coils represent α-helices. The stars indicate the active sites observed in this report for enzymatic reaction.