Table 1.
Optimization of APKR for the synthesis of dienone 10a.
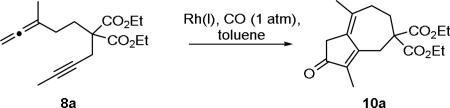
| ||||||
|---|---|---|---|---|---|---|
| Entry | Rh(I) | Catalyst Loading (mol%) | Temperature (°C) | Concentration (M) | Time | Yield (%) |
| 1 | [Rh(CO)2Cl]2 | 15 | 90 | 0.1 | 1.5 h | 27 |
| 2 | [Rh(CO)2Cl]2 | 15a | 90 | 0.1 | 1.5 h | 32 |
| 3 | [Rh(CO)2Cl]2 | 15a | 90 | 0.01 | 1.5 h | 53 |
| 4 | [Rh(CO)2Cl]2 | 15a | 110 | 0.01 | 25 min | 57 |
| 5 | [Rh(CO)2Cl]2 | 10a | 110 | 0.01b | c | 81 |
| 6 | [Rh(CO)2Cl]2 | 5a | 110 | 0.01b | c | 80 |
| 7 | [Rh(CO)2Cl]2 | 2a | 110 | 0.01b | c | 62 |
| 8 | [Rh(CO)2Cl]2 | 1a | 110 | 0.01b | c | 63 |
| 9 | [Rh(CO)(dppp)2]Cl | 10a | 110 | 0.1 | 21 hd | 27 |
| 10 | [Rh(CO)(dppp)2]Cl | 10a | 110 | 0.01 | 21 hd | 46 |
| 11 | [Rh(CO)Cl(dppp)]2 | 10a | 110 | 0.01 | 6 h | 29 |
Triphenylphosphine polymer bound was used in the reaction work up.
Syringe pump addition of allene-yne to Rh(I) solution was utilized.
Dropwise addition over 1.5 h and an additional 15 min of reaction time.
Reaction was not monitored between 8-21 h.
