Table 1.
RBF3K synthesis with and without the use of ethylene glycol additive.

| |||
|---|---|---|---|
|
| |||
| entry | product | X | % yield (time) a |
| 1 |
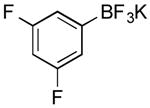
|
CI | 80 (20 min) |
| 77 (1.5 h) | |||
| 2 |
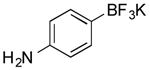
|
CI | 82 (2 h) |
| 68 (2 h) | |||
| 3 |
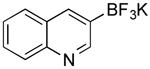
|
Br | 92 (20 min) |
| 68 (1 h) | |||
| 4 |
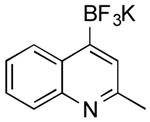
|
Cl | 52 b (30 min) |
| 47 (1.5 h) | |||
| 5 |
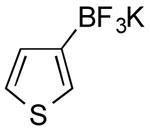
|
CI | 80 (3.5 h) |
| 0 | |||
| 6 |
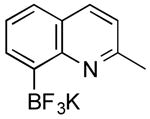
|
CI | 65 (1 h) |
| 52 (2 h) | |||
| 7 |
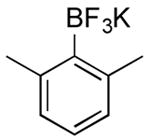
|
CI | 69 (1 h) |
| 53 (4.5 h) | |||
| 8 |
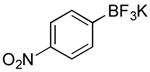
|
CI | 69 (25 min) |
| 64 (4 h) | |||
| 9 |
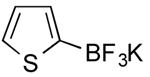
|
CI | 27 (45 min) |
| 0 | |||
| 5 c | |||
| 10 |
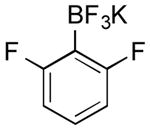
|
CI | 66 d (84 h) |
| 43 e | |||
| 37 f (22 h) | |||
| 0 g (3 h) | |||
| Br | 0 | ||
| 11 |
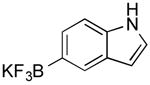
|
CI | 37 (50 min) |
| 12 |
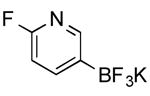
|
CI | 76 (3 h) |
| 73 h (75 min) | |||
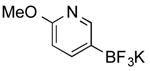
| 13 |
|
CI | 88 i (3 h) |
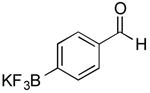
| 14 |
|
CI | 73 j (1 h) |
| 75k (4 h) | |||
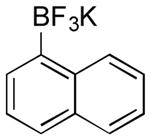
| 15 |
|
OMs | 60 (4 h) |
| 0 | |||
1.5 mmol scale unless otherwise noted.
As an inseparable mixture of internal salt and potassium trifluoroborate.
Using (Me2N)2B- B(NMe2)2 as the borylating agent.
0 °C.
rt.
50 °C.
80 °C.
65 °C, 7.6 mmol scale.
65 °C.
13% coupled alcohol reduction product was also generated.
30% coupled alcohol reduction product was also generated.
