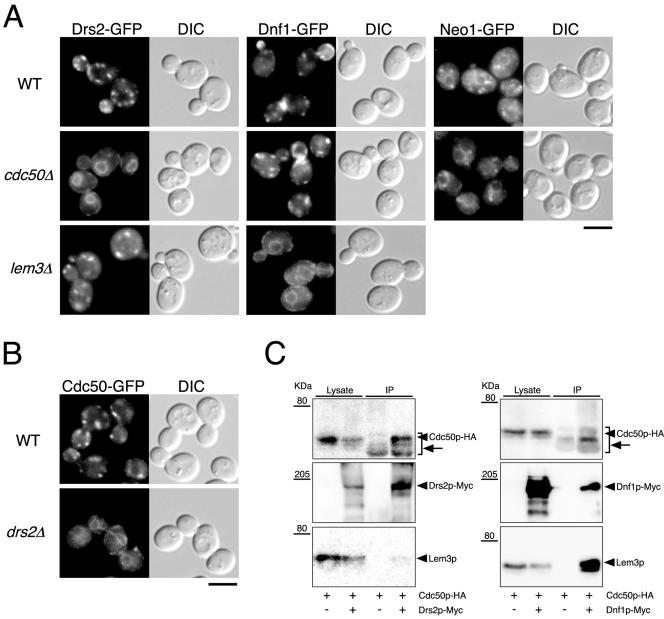
Figure 4.
Cdc50p-Drs2p and Lem3p-Dnf1p complex formation is required for the ER exit of these proteins. (A) Localization of Drs2p-GFP, Dnf1p-GFP, and Neo1p-GFP in wild-type, cdc50Δ, and lem3Δ cells. (B) Localization of Cdc50p-GFP in wild-type and drs2Δ cells. Cells were grown to early-mid logarithmic phase in YPDA medium at 30°C and observed immediately by microscopy. GFP-tagged proteins were visualized using a GFP bandpass filter. The strains used were as follows: YKT768 (Drs2p-GFP in WT), YKT769 (Drs2p-GFP in cdc50Δ), YKT770 (Drs2p-GFP in lem3Δ), YKT771 (Dnf1p-GFP in WT), YKT772 (Dnf1p-GFP in cdc50Δ), YKT773 (Dnf1p-GFP in lem3Δ), YKT775 (Neo1p-GFP in WT), YKT776 (Neo1p-GFP in cdc50Δ), YKT259 (Cdc50p-GFP in WT), YKT774 (Cdc50p-GFP in drs2Δ). Bars, 5 μm. (C) Coimmunoprecipitation of Cdc50p and Lem3p with Drs2 and Dnf1p, respectively, in sec12-4 cells. sec12-4 cells were grown at 25°C to mid logarithmic phase in YPDA medium and then incubated at 35°C for 1 h. Drs2p-Myc or Dnf1p-Myc was immunoprecipitated with anti-Myc antibody, and the precipitated proteins were detected by immunoblot analysis as described for Figure 3. Arrows indicate anti-Myc antibody detected by secondary antibodies (anti-mouse IgG-HRP). The strains used were as follows: YKT913 (Drs2p-Myc Cdc50p-HA sec12-4), YKT914 (Dnf1p-Myc Cdc50p-HA sec12-4), and YKT915 (Cdc50p-HA sec12-4).