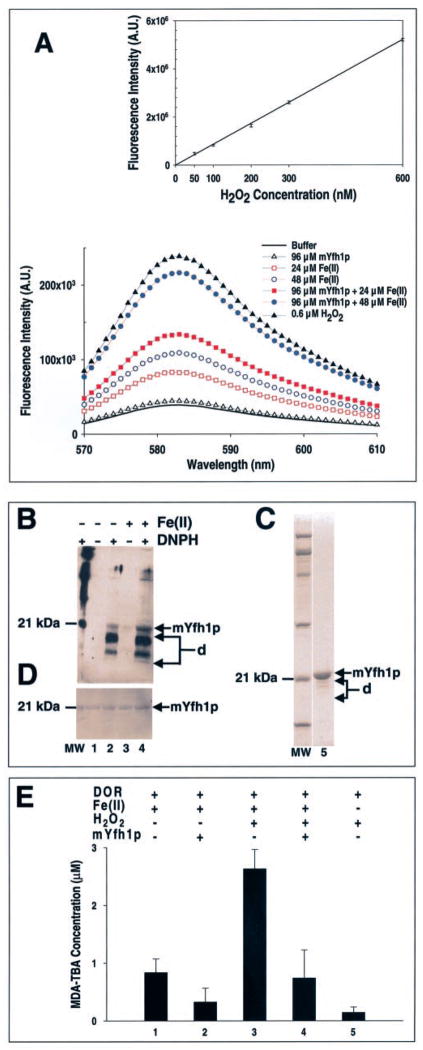
Fig. 3. Production of H2O2 during Fe(II) oxidation in mYfh1p.
A, reactions containing 96 μM mYfh1p and 24 or 48 αM Fe(II) were incubated in 50 mM HEPES-KOH, pH 7.0, for 30 min at 30 °C in the presence of Amplex Red/HRP reagent. Immediately afterward, fluorescence intensity was recorded from 570 to 610 nm. The fluorescence intensity curves before background correction are shown (bottom graph). For the standard curve (top graph), H2O2 standards (50 – 600 nM) were incubated in 50 mM HEPES-KOH, pH 7.0, for 30 min at 30 °C in the presence of Amplex Red/HRP reagent. The fluorescence intensity curves were recorded, and samples containing buffer plus Amplex Red/HRP were used for background corrections. The corrected fluorescence intensity curves were integrated, and a standard curve was constructed. The correlation coefficient of the fitted line to the data is 0.999. To determine the concentration of H2O2 produced in the presence of mYfh1p (see “Results”), samples containing buffer plus 24 or 48 μM Fe(II) and Amplex Red/HRP were used as blanks for background corrections. The corrected fluorescence intensity curves for mYfh1p were integrated, and the H2O2 concentration was calculated from the standard curve. A.U., arbitrary units. B, reactions containing 96 μM mYfh1p were incubated for 30 min at 30 °C in the absence or presence of 48 μM Fe(II) under the experimental conditions used in the Amplex Red/HRP assays described above. Following incubation with 2,4-dinitrophenylhy-drazine (DNPH) to derivatize carbonyl groups to DNP, the samples (7.5 μg of total protein) were analyzed by SDS/PAGE and Western blotting using a polyclonal anti-DNP antiserum. C, 2 μg of the purified mYfh1p monomer used in the experiments described above was analyzed by SDS/PAGE and Coomassie Blue staining (lane 5). D, following immunodetection, the membrane was subjected to SYPRO Ruby protein blot staining. Arrows d, degradation products of mYfh1p; lane MW, molecular mass standards. E, a mixture of 48 μM Fe(II), 24 μM H2O2, and 5 mM 2-deoxyribose (DOR) was incubated in 10 mM HEPES-KOH, pH 7.0, in the absence or presence of 96 μM mYfh1p for 30 min at 30 °C, and production of malondialdehyde-thiobarbituric acid (MDA-TBA) (ε532 = 1.54 × 105 M−1 cm−1) was measured (31). The indicated controls were analyzed at the same time and treated identically. The bars represent the means ± S.D. of four independent measurements.