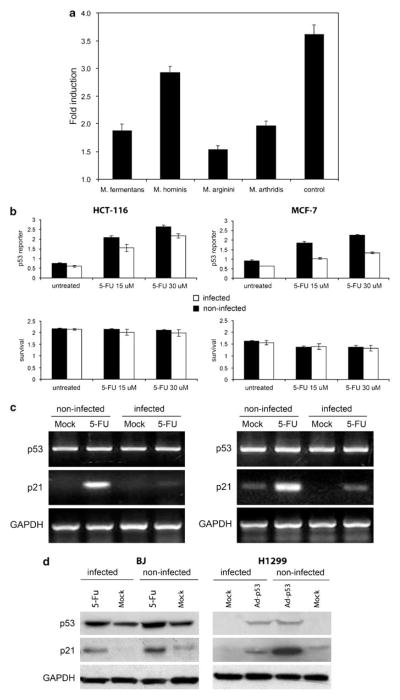
Figure 1.
Suppression of p53 transcriptional activity by mycoplasmas. (a) ConA-3T3 cells carrying a p53-dependent β-galactosidase (β-gal) reporter (ConALacZ) were treated overnight with 5% conditioned broths collected from cell-free cultures of the mycoplasma species indicated or with regular medium (‘control’). Doxorubicin was then added to a final concentration of 1 μg/ml and cells were cultured for an additional 24 h. The induction of β-gal activity in total cell lysates was determined by o-nitrophenyl β-D-galactopyranoside staining measured spectrophotometrically at 414 nm. The values plotted indicate the ratio of the β-gal activity in doxorubicin-treated cells infected with a given mycoplasma species to the β-gal activity in untreated cells infected with the same mycoplasma species. (b) p53-responsive reporter activity in control and M. arginini-infected HCT-116 and MCF-7 cells. HCT-116 and MCF-7 cells stably transfected with the ConALacZ reporter construct were plated into 96-well plates and incubated in medium containing the indicated concentration of 5-FU for 16 h. The cells were infected with M. arginini at least 2 weeks before the experiment. Presence of M. arginini in cell culture was confirmed by semiquantitative PCR vis-à-vis nuclear gene sequences. β-gal activity was measured by o-nitrophenyl β-D-galactopyranoside (ONPG) staining (upper panels) and cell survival was measured by methylene blue staining (bottom panels). Infected (M. arginini) cells (black bars), noninfected cells (white bars). (c) Reverse transcription (RT)–PCR analysis of p53 and p21 mRNA expression in control and M. arginini-infected HCT-116 and MCF-7 cells. Cells were left uninfected or were infected by growth in 5% M. arginini-conditioned medium overnight. To induce p53, cells were then treated with 5-FU (30 μM) for 24 h. Levels of p53 and p21 expression were measured by RT–PCR. Expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a positive control to confirm the quality and amount of cDNA in each sample. HCT-116 cells (left panel), MCF-7 cells (right panel). (d) Effect of M. arginini infection on p53 and the p53-transcriptional target, p21. Lysates from control (noninfected) and M. arginini-infected BJ and H1299 cells were used for western blot analysis of p53 and p21 protein levels. GAPDH expression was used to control for protein concentration between lanes. To induce p53 activity, BJ cells were treated with 30 μM 5-FU for 16 h and H1299 cells were treated with 10 PFU (plaque-forming units) of Ad-p53 per cell.