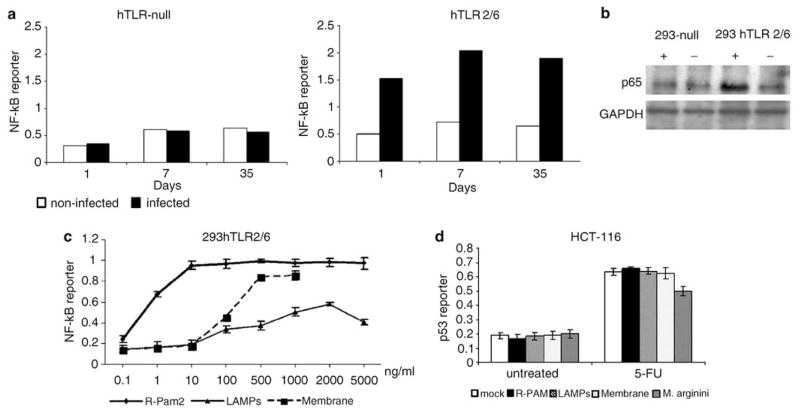
Figure 2.
Activation of nuclear factor (NF)-κB-dependent transcription in cells infected with M. arginini. (a) N F-κB-responsive reporter activity in control and M. arginini-infected 293hTLR-null cells (not expressing any Toll-like receptors (TLRs)) and 239hTLR2/TLR6 cells (expressing hTLR2 and TLR6 but not any other TLRs). 293hTLR-null and 239hTLR2/TLR6 cells bearing the NF-κB-LacZ reporter construct were infected with M. arginini. Next, cells were plated into 96-well plates and β-galactosidase (β-gal) activity was measured by o-nitrophenyl β-D-galactopyranoside (ONPG) staining 1, 7 and 35 days later. Infected cells (black bars), noninfected cells (white bars). (b) Influence of M. arginini infection on the nuclear localization of the p65 subunit of NF-κB. 293hTLR-null and 239hTLR2/TLR6 cells infected (+) or noninfected (−) with mycoplasma were collected 40 days post-infection and lysed for extraction of nuclear proteins. p65 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (control for equal protein loading) were detected by western blotting. (c) Effect of different mycoplasma components on NF-κB activity. N F-κB-responsive reporter activity in 293 cells expressing hTLR2/hTLR6 treated with different concentrations of mycoplasmal membranes, lipid-associated mycoplasmal proteins (LAMPs) and synthetic analogs of mycoplasmal MALP-2 lipopeptides (R-PAM2). (d) Induction of p53-responsive reporter activity in HCT-116 ConALacZ cells left untreated (‘mock’), treated with mycoplasmal membranes (‘membrane,’ 1μg/ml), lipid-associated membrane proteins (‘LAMPs,’ 2 μg/ml) or synthetic analogs of mycoplasmal MALP-2 lipopeptides (‘R-PAM,’ 0.1 μg/ml) or infected with M. arginini. p53 reporter activity was determined in cells grown in 15 μM 5-FU for 24 h and in cells grown without 5-FU (‘negative control’).