Abstract
Mutations in the gene encoding dysferlin cause two distinct muscular dystrophy phenotypes: Limb-girdle muscular dystrophy type 2B (LGMD-2B) and Miyoshi myopathy (MM). Dysferlin is a large transmembrane protein involved in myoblast fusion and membrane resealing. Zebrafish represent an ideal animal model to use for studying muscle disease including abnormalities of dysferlin. cDNAs of zebrafish dysferlin were cloned (6.3 kb) and the predicted amino acid sequences, including abnormalities of dysfelin, showed 65% similarity to predicted amino acid sequences of mammalian dysferlin. The expression of dysferlin was mainly in skeletal muscle, heart and eye, which could be detected as early as 11 hours post fertilization (hpf). Three different antisense oligonucleotide morpholinos were targeted to inhibit translation of this dysferlin mRNA and the morpholino-injected fish showed marked muscle disorganization which could be detected by birefringence assay. Western blot analysis using two different dysferlin antibodies showed that the expression of dysferlin was reduced in each of the three morphants. Dysferlin expression was shown to be reduced at the myosepta of zebrafish muscle using immunohistochemistry, although the expression of other muscle membrane components, dystrophin, laminin, beta-dystroglycan were detected normally. Our data suggest that zebrafish dysferlin expression is involved in stabilizing muscle structures and its downregulation causes muscle disorganization.
INTRODUCTION
Mutations in dysferlin cause clinically distinct forms of muscular dystrophies, limb girdle muscular dystrophy 2B (LGMD2B) [1], Miyoshi Myopathy (MM) [2] and distal anterior compartment myopathy [3]. Many cases of muscular dystrophy in humans are caused by mutations in the dystrophin associated protein complex (DAPC). However, in dysferlin-null muscle cells, degeneration is mediated through a mechanism distinct from that of the DAPC-linked dystrophies. Dysferlin is a large transmembrane protein (237 kDa) involved in the resealing of membrane tears and the fusion of myoblasts during myogenesis [4]. Dysferlin has also been shown to interact with several cytosolic and membrane-associated proteins, such as MG53, affixin, annexins A1 and A2, AHNAK, caveolin-3 and calpain-3 [5], [6], [7], [8], [9], [10]. Dysferlin is a large type II transmembrane protein composed of multiple C2 domains and two Dysf domains [11]. Dysferlin’s highly conserved C2 domains are thought to play a crucial role in calcium phospholipid binding during vesicle trafficking and membrane patch repair [4], [12], [13], [14].
Zebrafish have been used to study a number of other mammalian forms of muscular dystrophy including dystrophin deficiency, the cause of human Duchenne muscular dystrophy, as well as laminin deficiency causative of CMD [15–21]. The analysis of these human disorders in zebrafish has been facilitated by their transparency early in development, rapid development and clear skeletal muscle abnormalities detectable upon birefringence. They have also been used to rapidly screen for small molecules, which might modulate the affects of these mutations in muscle [22]. They are also easily manipulated via the use of morpholinos during development.
To start to make a fish model of dysferlinopathy for studies on its function and for therapeutic drug screens, we first identified the dysferlin gene in zebrafish and analyzed its expression in zebrafish. Once confirming the identity of zebrafish dysferlin, morpholinos that targeted zebrafish dysferlin mRNA were designed and injected into zebrafish eggs. The resulting decrease in dysferlin expression caused clear disorganization of skeletal muscle as well as abnormalities of brain and eye development. Our data indicates the reduction of dysferlin expression causes the abnormal formation of muscle structures.
MATERIALS AND METHODS
Fish and fish culture
Eggs derived from AB fish matings were used for injection. Zebrafish embryos were collected and raised at 28.5°C according to standard procedures [23] and standard criteria [24] under the guidelines of our Institutional Animal Care and Use Committee.
Cloning of zebrafish dysferlin cDNA
Zebrafish total RNA was extracted from 4 dpf wild type embryos, purified with the RNeasy micro kit (QIAGEN), and converted to cDNA using SuperScript III first-strand system for RT-PCR (Invitrogen) according to the manufacturer’s protocol. Primers used to amplify the full-length fish dysferlin cDNA coding sequence were forward; 5′-TTGCAGACAAGTGTTACTAGTGCCGATCCTGCTGT-3′, reverse; 5′-TCACTGTGTTCCCTTTCCTAGTCCACTGAAGGGTCC -3′. To construct fish dysferlin mRNAs with myc tag constructs, Primers used for PCR were forward; 5′-ATCGATATGCTGCGTGTGTTTATTCTGTGCGCCCAGAATGTGCTGACGCATGATGAGGACATT-3′, reverse; 5′-GCGATCGATTCACAGATCCTCTTCTGAGATGAGTTTTTGTTCCTGTGTTCCCTTTCCTAGTCCACTGAAGGGTCC-3′. PCR was performed with Phusion Hot Start High-Fidelity DNA Polymerase (New England Biolabs) at 95 °C for 30 sec, 60°C for 30 sec, 72°C for 5 min (35 cycles) according to the manufacturer’s protocol. The PCR product of the zebrafish dysferlin cDNA was cloned into Strata clone PCR Cloning vector (pSC-A) (Stratagene). The dysferlin gene sequence was confirmed by sequence analysis and the cDNA was then subcloned into pCS2+ after digestion with ClaI (New England Biolabs). All PCR products and cloned fragments were sequenced by the Molecular Genetics Core Facility at Children’s Hospital Boston using sequencing primers for dysferlin sequence analysis (supplemental data 1). Using NCBI database and program on BLAST sequencing results were analyzed.
RNA expression analysis via RT-PCR
To analyze the expression of dysferlin during early development, zebrafish total RNA was extracted from 1 cell stage, 2, 4, 11, 24, 48, 72 and 96 hpf wild type embryos. To analyze the expression of dysferlin in the different tissues, zebrafish total RNA was extracted from brain, eye, heart, skeletal muscle, gut and liver of wild type adult fish (4 month old fish) and converted to cDNA as described above. RNA expression analysis was accomplished using PCR with ExTaq DNA Polymerase (Takara) at 95 °C for 30 sec, 60°C for 30 sec, 72°C for 1 min (35 cycles) with primer sets of zebrafish dysferlin, forward; 5′-TTGCAGACAAGTGTTACTAGTGCCGATCCTGCTGT-3′, reverse; 5′-CCCCAGTGCTGTTTCTCTTT-3′, primer sets of zebrafish β-actin used as a control; forward; 5′- ATCAGCATGGCTTCTGCTCT-3′, reverse; 5′-CACCCTGGCTTACATTTTCAA -3′.
PCR products were separated by electrophoresis on 1% agarose gels to analyze the expression.
For analysis of expression in different tissues, mRNA was extracted from zebrafish adult brain, eyes, heart, skeletal muscle, gut and liver using RNeasy micro kit (Qiagen). Two hundred nanograms of total RNA per sample was converted into cDNA using the FirstStrand Synthesis kit (Invitrogen). Ten-fold serial dilutions of the cDNA were analyzed on 96-well optical plates using the Power SYBR Green Mix system (Applied Biosystems) and analyzed on the ABI 7900HT real time PCR machine (Applied Biosystems) following the manufacturer’s instructions using with primer sets of zebrafish dysferlin; specific forward for known zebrafish dysferlin cDNA (XM684324); 5′-AGAGCGACCAACCTGAGAAA-3′, specific forward for cDNA of zebrafish dysferlin with different alternative n-terminus; 5′-ACTAGTGCCGATCCTGCTGT -3′, reverse for each different forward primers; 5′-CTCCCCCAGAAACCTATTCC -3′, primer sets of zebrafish elongation factor 1(EF1) α; forward; 5′-CTGGAGGCCAGCTCAAACAT-3′, reverse; 5′-ATCAAGAAGAGTAGTACCGCTAGCATTAC -3′. Cycle times were normalized to EF1α as a loading control. Relative fold expression and changes were calculated using the 2−ΔΔCt method.
Generation of expression constructs and in vitro transcription of RNAs
Fish dysferlin mRNAs with myc tag constructs were cloned into the pCS2+ expression vector. RNAs were synthesized from Asp718-digested pCS2+ plasmids using the sp6 mMessage mMachine kit (Ambion). The RNAs were quantified by nano drop (Thermo scientific). Phenol red (0.1%) was added to the RNA solution as a tracer, and mRNAs (100 pg) were co-injected into 1-cell-stage embryos with dysferlin MO2. Following injection, embryos were cultured in aquatic system at 28.5 °C.
Detection of muscle phenotype of dysferlin morphants by birefringence
Abnormal birefringence of muscle was observed and analyzed by placing anesthesized embryos on a glass-polarizing filter and subsequently covering them with a second polarizing filter. The filters were placed on an underlit dissection scope (Nikon, SMZ1500) and the top-polarizing filter twisted until only the light refracting through the striated muscle was visible. Since the degree of birefringence is affected by the horizontal orientation of the fish, the fish were oscillated back and forth to account for differences in positioning.
Morpholino oligonucleotide injections
Three different non-overlapping anti-sense morpholino oligonucleotides (MO) (Gene Tools LLC) targeted to interfere with fish dysferlin translation were designed using the 5′ sequence around the putative translation start site of the cloned zebrafish dysferlin mRNA. The morpholino sequences were dysferlin MO1: 5′-ATGCTGCGTGTGTTT ATTCTGTGCG-3′, MO2: 5′-ATCCTGCTGTTACCACTGTCTACCA-3′ and MO3: 5′-TTGCAGACAAGTGTTACTAGTGCCG-3′. Morpholinos were re-suspended in 1x Danieau solution [58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca (NO3)2, 5 mM HEPES; pH 7.6] with 0.1% phenol red as an injection indicator. Morpholinos (1.5 ng, 3 ng, 6 ng and 12 ng) were injected into the yolk of one- to two-cell stage embryos.
Immunohistochemistry
For immunohistochemical staining of whole mutant and morphant fish bodies, embryos were fixed in 100% methanol overnight at 4 °C and subsequently stored at −20 °C. Following rehydration with a methanol series (70–50% and PBS-T) and blocking with 2 % casein, 0.1% Tween 20 in PBS to reduce non-specific binding, embryos were incubated separately with either anti-dysferlin (1:20, Vector laboratories), anti-dystrophin (1:25, Sigma), anti-laminin (1:25, Sigma), anti-beta dystroglycan (1:100, Novocastra), or anti-myosin heavy chain (1:50, hybridoma bank) antibodies at 4 °C overnight. After washing several times, the samples were incubated with secondary antibodies (1:500, anti-mouse AlexaFluor 488, 1:500, anti-rabbit AlexaFluor 568, Invitrogen) for 30 min. The stained embryos were observed under a Nikon Eclipse E-1000 microscope, and photographed using a Hamamatsu digital camera. Images were acquired using Openlab software version 3.1.5 (Improvision).
Western Blotting
Embryos or fish were homogenized in RIPA buffer (Sigma) containing protease inhibitors and phosphatase inhibitors (Roche). Following centrifugation at 14000 × g for 20 min, the concentration of protein in the supernatants was determined using a protein assay kit (Bio-rad). Proteins were separated by electrophoresis on 4–12% gradient Tris-glycine gels (Invitrogen) and transferred onto a PVDF membrane (Invitrogen). After blocking the membrane in PBS containing 2% casein, 0.05% Tween 20, blotted proteins were incubated with primary antibody, anti dysferlin (1:100, Vector laboratories) or anti β-actin (1:500, Sigma) at 4 °C overnight. After washing, the membranes were incubated with horseradish peroxidase secondary antibody (anti-rabbit or mouse IgG, 1:15000, Invitrogen). Proteins were detected using a Western blotting detection kit (Millipore).
RESULTS
cDNA cloning of zebrafish dysferlin
A 6.3kb of zebrafish cDNA (6,344bp) was cloned from zebrafish total RNA. The predicted amino acid sequence had approximatly 68% similarity to that of mammal dysferlin (supplemental data 3). The sequences of the n-terminal region of this zebrafish dysferlin cDNA were different from those of other fish dysferlin cDNAs (XM684324) which had already been reported (supplemental data 3, Fig. 1A). In particular, the n-terminal region of the predicted amino acid sequences was markedly similar to human dysferlin variant 8 (NM003494) and mouse dysferlin variant 2 (NM001077694) in mammals (Fig. 1A). The new variant of zebrafish dysferlin has six C2 domains as well as Fer A and Fer B domains of already registered zebrafish dysferlin in NCBI database, which are known to be specific domains of dysferlin (underlined in supplemental data 3). The transmembrane domain was also present in the c-terminal region.
Figure 1.
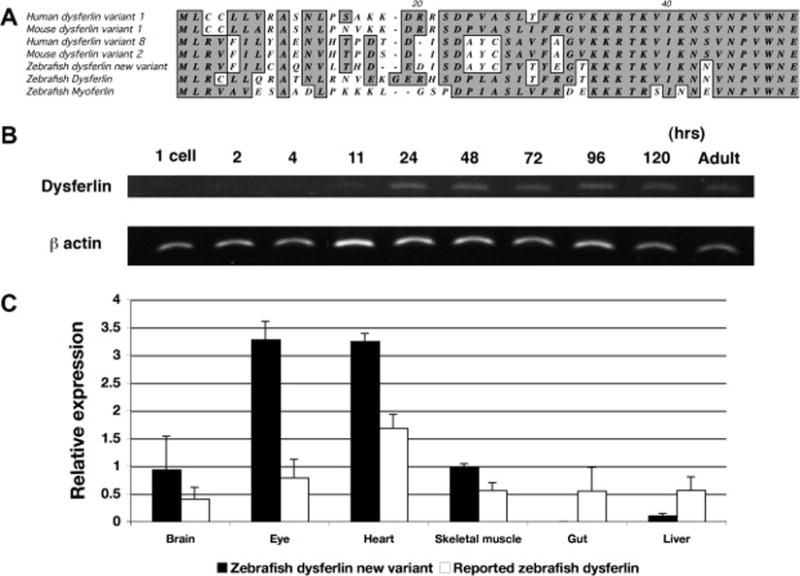
Expression of zebrafish dysferlin
A, Alignment of n-terminal region of the predicted amino acid sequences of dysferlin and myoferlin
B, expression analysis at different developmental stages, 1 cell stage, 2, 4, 11, 24, 48, 72 and 96 hpf by RT-PCR. C, expression analysis of dysferlin alternative amino terminal domains in different tissues, adult brain, eyes, heart, skeletal muscle, gut and liver by quantitative real time PCR. White bars show relative expression level of the previously reported dysferlin amino terminus, black bars show relative expression level of new dysferlin alternative amino terminus.
Expression of zebrafish dysferlin
The two alternative amino terminal domains of zebrafish dysferlin were analyzed by PCR of different zebrafish mRNA fraction using the same internal reverse PCR primer. Analysis of mRNA prepared from different times during zebrafish development revealed that zebrafish dysferlin first appears at 11 hours post fertilization (Fig. 1B) and continues to be expressed until 120 hours post fertilization (Fig. 1B). The dysferlin mRNA was found to be expressed in a number of different tissues including muscle and quite predominantly in brain and the eye (Fig. 1C). Both amino-terminal isoforms were expressed in the same tissues but the new variant seemed to be more highly expressed in the brain than the other isoform (Fig. 1C).
Dysferlin morphant morphology
To begin the study of the function of dysferlin in zebrafish embryo development, three different anti-sense morpholino oligonucleotides (MO) 1, 2 and 3 were designed to disrupt the translation of dysferlin mRNA during development. Following injection of the three different morpholinos there were clear developmental abnormalities visible upon light microscopy. To better visualize the structure and organization of muscle fibers of the morphants, dysferlin morphants, control embryos and wild type embryos were analyzed by birefringence assays at 4 dpf (Fig. 2A). Dysferlin morphant embryos were found to have markedly reduced normal patterns of birefringence compared to wild type and control MO (Fig. 2A).
Figure 2.
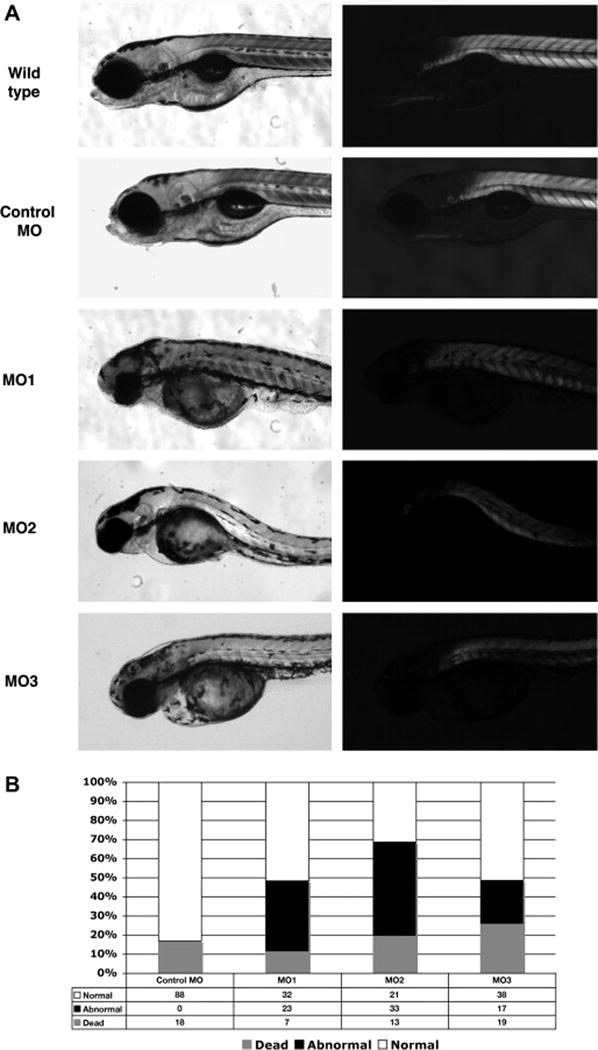
Dysferlin morphants at 4 dpf
A, Dysferlin morphant at 4 dpf (Wild type fish, control MO injected fish, dysferlin MO1, MO2 and MO3 injected fish). Left, bright field images. Right, birefringence assay. B, Histogram of the percentage of normal, dead and affected fish in MO1, 2 or 3-injected fish. White bar shows the percentage which appeared normal. Black indicates the percentage of fish with abnormal development and gray indicated the percentage of fish which were dead.
There were also marked abnormalities of both brain and eye development in these morpholino treated zebrafish. These experiments were done on 3 separate occasions and very similar results were obtained on each experiment. As has been observed in other morpholino experiments there were both live normal, as well as dead fish, observed following morpholino injections.
Injection of 6 ng of MO1, MO2 or MO3 resulted in approximately 37.1%, 49.3% and 23% of injected fish exhibiting muscle phenotypes (as detected by birefringence assay), 51.6%, 31.3% and 51.4% of them were normal and 11.3%, 19.4% and 25.7% of them were dead respectively (Fig. 2B). These are an averaging of the results over three different experiments.
To confirm the knock down of the expression of fish dysferlin with morpholino injection, endogenous dysferlin protein was analyzed with antibodies originally designed against human dysferlin. Dysferlin expression was clearly detected at the myosepta in wild type and control MO injected fish, however the dysferlin expression in individual dysferlin morpahnt 1, 2 and 3 was markedly reduced at the myosepta of injected zebrafish compared to those of wild type and control MO injected fish (Fig. 3A). These immunohistochemistory results were replicated with a different dysferlin antibody (data not shown). In wild type fish and control MO injected fish, the dysferlin antibody recognized 240 kDa protein on western blots, a size predicted by the fish dysferlin sequence. The analysis of morphant 1, 2 and 3 extracts showed that the expression of dysferlin protein in morphants was substantially decreased compared to those of wildtype fish and control MO-injected fish (Fig. 3B). The western blot results show that the morpholinos used in these injections were effective in disturbing the expression of dysferlin during zebrafish development.
Figure 3.
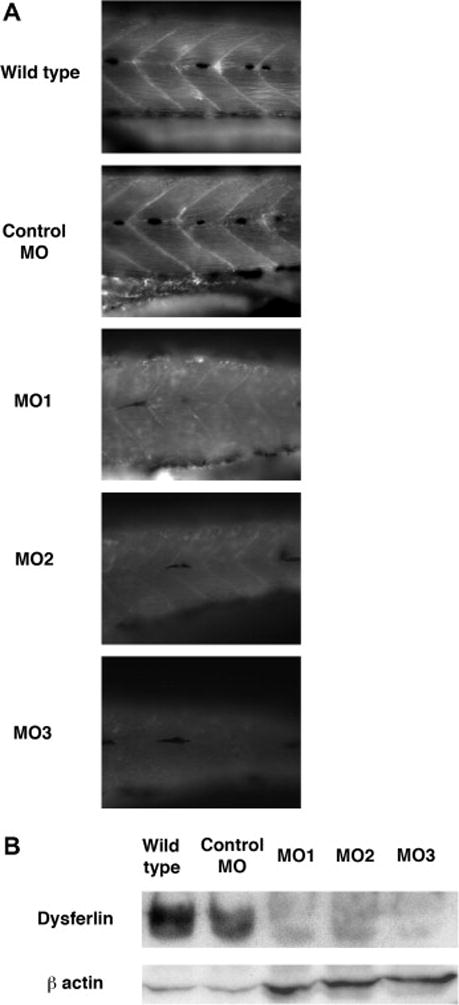
Immunostaining of dysferlin morphant with antibodies against human dysferlin. A, Immunostaining of dysferlin morphant. Left panels, the results of immunostaing with anti-dysferlin in wild type, control MO, dysferlin MO1, 2 and 3. Right panels, the results with the anti-laminin antibody in wild type, control MO, dysferlin MO1, 2 and 3. B, Immunoblot of protein extracts from wildtype fish, fish injected with control MO, dysferlin MO1, MO2 and MO3 with anti-dysferlin. β-actin was detected as an internal control.
Immunohistochemistory of dysferlin morphants with muscle structure’s components
To examine the expression of other muscle proteins, antibodies against dystrophin, laminin, β-dystroglycan and myosin heavy chain (MHC, slow fiber) were used for immunohistochemistry on the morphant embryos. Dystrophin, laminin and β-dystroglycan expression at the myosepta appeared normal (Fig. 4), however the immunostaining of these antibodies indicated that somite boundaries were misshapen and had a less clear v-shaped structure as seen in controls (Fig. 4). Staining with anti-MHC indicated that formation of myofibers was disturbed (arrows in fig. 4).
Figure 4.
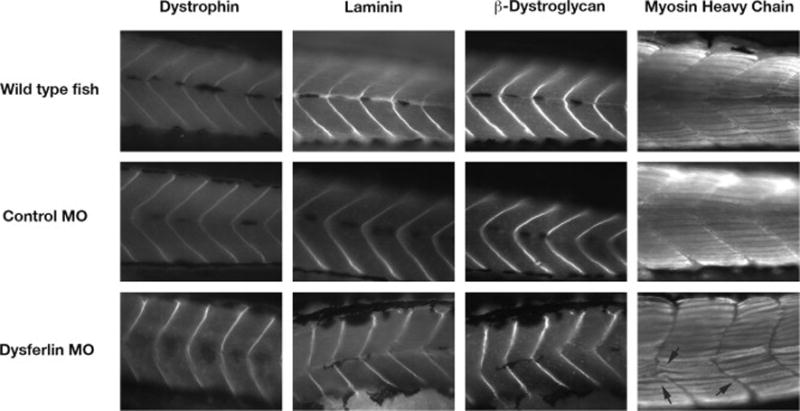
Immunostaining of dysferlin morphant zebrafish with antibodies against different muscle protein components, dystrophin, laminin, β-dystroglycan and myosin heavy chain in wild type, control MO, dysferlin MO2. Arrows indicate the disturbance of myosepta in the dysferlin morphant.
DISCUSSION
A cDNA of the zebrafish dysferlin alternative amino-terminal domain to that present in the NCBI database was cloned and the predicted amino acid sequences had high similarity to mammalian dysferlin. It has the same six C2 domains, a Fer A and a Fer B domain and a transmembrane domain, which are known as the dysferlin specific domains in mammals. The sequences of both zebrafish dysferlin sequences are more similar to mammalian dysferlin than those of mammalian myoferlin. In human, many variants of dysferlin are present, from variant 1 to variant 14. In the n-terminal region, these predicted amino acid sequences were very similar to those of human dysferlin variant 8 and mouse dysferlin variant 2. The sequences of this zebrafish dysferlin variant are nearly identical to those of the already reported zebrafish dysferlin except for n-terminal region, which indicates that this dysferlin may be the alternative amino-terminus of zebrafish dysferlin known to be present in mammals.
The expression of dysferlin mRNA was clearly detected in skeletal muscle, heart and eye. During development, the expression of dysferlin was detected from 11 hpf. It is known that the first somite in zebrafish is formed around 10–11 hpf [23, 24], which suggests that the expression may play important roles for the development of the skeletal muscle. When dysferlin expression is suppressed during development by the use of 3 different morpholinos, zebrafish development is clearly abnormal. Not only did skeletal muscle show distinct abnormalities detected by both birefringence as well as immunohistochemistry, there was also a marked abnormality of brain and eye development.
Other skeletal muscle components such as, dystrophin, laminin, β-dystroglycan and myosin heavy chain were normally localized, however muscle structure and formation of myofibers were disturbed when dysferlin expression is reduced. These findings indicate that dysferlin has a function in the development of normal muscle structure not linked to expression of these other proteins and may be mediated through a mechanism distinct from that of the DAPC-linked dystrophies in zebrafish.
Supplementary Material
Supplemental data 1 primers for sequence analysis.
Supplemental data 2. cDNA of zebrafish dysferlin.
Supplemental data 3. Alignment of predicted amino acid sequences of zebrafish dysferlin.
Acknowledgments
We would like to acknowledge members of the Kunkel lab for their experimental advice, help in performing these experiments and the drafting of this manuscript. In addition, we are grateful to Chris Lawrence and Jason Best who managed our fish facility. This work was supported by a grant from NINDS # 5P50NS040828-09, and the Muscular Dystrophy Association and Jain foundation. All sequencing was accomplished in the IDDRC Molecular Core Laboratory supported by NICHD # 2P30HD018655-26.
Footnotes
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest in regard to this manuscript for any of the authors.
References
- 1.Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, et al. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet. 1998;20:37–42. doi: 10.1038/1689. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Aoki M, Illa I, Wu C, Fardeau M, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet. 1998;20:31–36. doi: 10.1038/1682. [DOI] [PubMed] [Google Scholar]
- 3.Illa I, Serrano-Munuera C, Gallardo E, Lasa A, Rojas-Garcia R, et al. Distal anterior compartment myopathy: a dysferlin mutation causing a new muscular dystrophy phenotype. Ann Neurol. 2001;49:130–134. [PubMed] [Google Scholar]
- 4.Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda C, Kameyama K, Tagawa K, Ogawa M, Suzuki A, et al. Dysferlin interacts with affixin (beta-parvin) at the sarcolemma. J Neuropathol Exp Neurol. 2005;64:334–340. doi: 10.1093/jnen/64.4.334. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Lennon NJ, Kho A, Bacskai BJ, Perlmutter SL, Hyman BT, et al. Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J Biol Chem. 2003;278:50466–50473. doi: 10.1074/jbc.M307247200. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Laval SH, van Remoortere A, Baudier J, Benaud C, et al. AHNAK, a novel component of the dysferlin protein complex, redistributes to the cytoplasm with dysferlin during skeletal muscle regeneration. Faseb J. 2007;21:732–742. doi: 10.1096/fj.06-6628com. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda C, Hayashi YK, Ogawa M, Aoki M, Murayama K, et al. The sarcolemmal proteins dysferlin and caveolin-3 interact in skeletal muscle. Hum Mol Genet. 2001;10:1761–1766. doi: 10.1093/hmg/10.17.1761. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Verheesen P, Roussis A, Frankhuizen W, Ginjaar I, et al. Protein studies in dysferlinopathy patients using llama-derived antibody fragments selected by phage display. Eur J Hum Genet. 2005;13:721–730. doi: 10.1038/sj.ejhg.5201414. [DOI] [PubMed] [Google Scholar]
- 10.Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, et al. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem. 2009;284:15894–15902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Therrien C, Dodig D, Karpati G, Sinnreich M. Mutation impact on dysferlin inferred from database analysis and computer-based structural predictions. Journal of the Neurological Sciences. 2006;250:71–78. doi: 10.1016/j.jns.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 12.McNeil AK, Rescher U, Gerke V, McNeil PL. Requirement for annexin A1 in plasma membrane repair. J Biol Chem. 2006;281:35202–35207. doi: 10.1074/jbc.M606406200. [DOI] [PubMed] [Google Scholar]
- 13.Mellgren RL, Zhang W, Miyake K, McNeil PL. Calpain is required for the rapid, calcium-dependent repair of wounded plasma membrane. J Biol Chem. 2007;282:2567–2575. doi: 10.1074/jbc.M604560200. [DOI] [PubMed] [Google Scholar]
- 14.Mellgren RL, Miyake K, Kramerova I, Spencer MJ, Bourg N, et al. Calcium-dependent plasma membrane repair requires m- or mu-calpain, but not calpain-3, the proteasome, or caspases. Biochim Biophys Acta. 2009;1793:1886–1893. doi: 10.1016/j.bbamcr.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steffen LS, et al. Zebrafish orthologs of human muscular dystrophy genes. BMC Genomics. 2007;8:79. doi: 10.1186/1471-2164-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassett DI, Currie PD. The zebrafish as a model for muscular dystrophy and congenital myopathy. Hum Mol Genet. 2003;12:265–270. doi: 10.1093/hmg/ddg279. [DOI] [PubMed] [Google Scholar]
- 17.Guyon JR, et al. Delta-sarcoglycan is required for early zebrafish muscle organization. Exp Cell Res. 2005;304:105–115. doi: 10.1016/j.yexcr.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Guyon JR, et al. The dystrophin associated protein complex in zebrafish. Hum Mol Genet. 2003;12:601–615. [PubMed] [Google Scholar]
- 19.Guyon JR, et al. Genetic isolation and characterization of a splicing mutant of zebrafish dystrophin. Hum Mol Genet. 2009;18:202–211. doi: 10.1093/hmg/ddn337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawahara G, Guyon JR, Nakamura Y, Kunkel LM. Zebrafish models for human FKRP muscular dystrophies. Hum Mol Genet. 2010;19:623–633. doi: 10.1093/hmg/ddp528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta V, Kawahara G, Gundry SR, Chen AT, Lencer WI, Zhou Y, Zon LI, Kunkel LM, Beggs AH. The zebrafish dag1 mutant: A novel genetic model for dystroglycanopathies. Hum Mol Genet. 2011;20:1712–1725. doi: 10.1093/hmg/ddr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawahara G, Karpf JA, Myers JA, Alexander MS, Guyon JR, Kunkel LM. Drug screening in a zebrafish model of Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2011;108:5331–6. doi: 10.1073/pnas.1102116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nusslein-Volhard C, Dahm R. Zebrafish: a Practical Approach. Oxford University Press; Oxford; 2002. [Google Scholar]
- 24.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data 1 primers for sequence analysis.
Supplemental data 2. cDNA of zebrafish dysferlin.
Supplemental data 3. Alignment of predicted amino acid sequences of zebrafish dysferlin.


