Abstract
The tumor microenvironment is increasingly recognized as a major factor influencing the success of therapeutic treatments and has become a key focus for cancer research. The progressive growth of a tumor results in an inability of normal tissue blood vessels to oxygenate and provide sufficient nutritional support to tumor cells. As a consequence the expanding neoplastic cell population initiates its own vascular network which is both structurally and functionally abnormal. This aberrant vasculature impacts all aspects of the tumor microenvironment including the cells, extracellular matrix, and extracellular molecules which together are essential for the initiation, progression and spread of tumor cells. The physical conditions that arise are imposing and manifold, and include elevated interstitial pressure, localized extracellular acidity, and regions of oxygen and nutrient deprivation. No less important are the functional consequences experienced by the tumor cells residing in such environments: adaptation to hypoxia, cell quiescence, modulation of transporters and critical signaling molecules, immune escape, and enhanced metastatic potential. Together these factors lead to therapeutic barriers that create a significant hindrance to the control of cancers by conventional anticancer therapies. However, the aberrant nature of the tumor microenvironments also offers unique therapeutic opportunities. Particularly interventions that seek to improve tumor physiology and alleviate tumor hypoxia will selectively impair the neoplastic cell populations residing in these environments. Ultimately, by combining such therapeutic strategies with conventional anticancer treatments it may be possible to bring cancer growth, invasion, and metastasis to a halt.
Keywords: Microenvironment, stem cells, metastases, hypoxia modifiers, vascular targeting, exercise
1.0. Introduction
In the development of a cancer, the transformation into a neoplastic and progressively invasive tumor occurs though a multitude of etiologies. Oncogenic lesions, coupled with inhibition of tumor suppressors, together contribute to cellular transformation. Genomic, proteomic, post-translational, and epigenetic mutations are responsible for activating oncogenes and inhibiting tumor suppressor genes. Several essential characteristics are present in malignancies; a key element of malignant transformation being the loss of regulatory control mechanisms. Cancer cells not only possess heightened rates of cell proliferation and aberrant cell cycle checkpoints, but also lose contact-inhibited growth regulation.
The developing tumor contains a distinct cellular compartment that retains stem-like cell characteristics, namely multipotency, self-renewal and proliferation potential, and this in turn drives oncogenesis and tumor progression. Thus a discrete subset of cells within a tumor possesses the capability of self-renewal and multipotency that gives rise to a heterogeneous population of cancer cells. Although cancer stem cells can vary by definition, they generally comprise less than 10% of the population of a tumor. Still, cancer stem cells have been implicated as a key component in the formation and spread of human cancers to distant sites.
In addition to the variety of neoplastic cells, many other cell types are present in the tumor milieu. Examples include fibroblasts, endothelial cells, hematopoietic-derived cells, and immune cells, which normally monitor this environment for foreign bodies. In cancer, particularly at the later stages of transformation and invasion, normal immune functions are subverted, leading to recognition of the tumor as part of the host, rather than as an invading foreign entity.
This cellular heterogeneity coupled with key physical and structural tissue components which form the extracellular matrix define the tumor microenvironment. The matrix is deposited as a mix of such proteins as collagens, fibronectin, laminins, hyaluronan, plasminogens, proteases, and numerous others which collectively form an inflexible scaffold to which cells attach. In addition, other secreted cellular proteins such as cytokines and extracellular matrix remodeling proteins normally reside in the extracellular matrix and contribute to its turnover regulation.
The tumor microenvironment is not static, but rather highly variable depending on tumor type and stage of cancer development. A critical factor impacting variations in the tumor microenvironment is the tumor supportive blood vessel network. The abnormal tumor vasculature and its physiologic consequences serve as an over-arching modulator of the tumor microenvironment affecting the composition and interaction of its constituents to impact tumor progression, cell dissemination, response to anticancer therapeutics, and cancer patient outcomes.
The deleterious effects of the tumor microenvironment on cancer therapy outcomes were first recognized in the 1950s when the possible negative consequences of tumor hypoxia for radiotherapy efficacy were postulated. Unfortunately, this issue has not been resolved. Indeed it has become abundantly clear that the effect of the tumor microenvironment is far more insidious than initially anticipated; now known to impact all conventional anticancer therapies, fundamental cancer cell biology, gene expression, and metastatic incidence. This review provides a historical context to our current perspectives of interventions strategies seeking to overcome the negative therapeutic consequences of the aberrant tumor microenvironment.
2.0. Physiological characteristics of the tumor microenvironment
2.1. Aberrant vascular networks
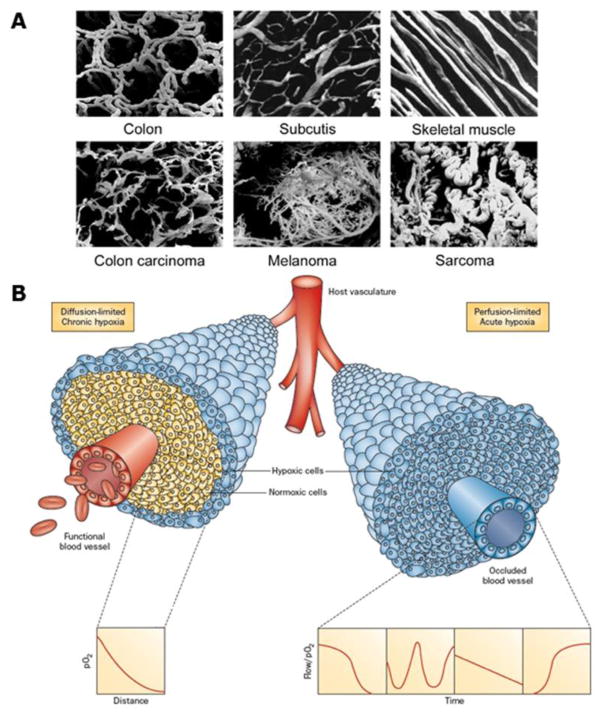
Survival of tumor cells and subsequent tumor development requires an adequate supply of oxygen and nutrients. These are initially supplied from the host vascular system, but the demand for these critical factors soon exceeds the supply from this source, thus tumors develop their own functional vascular supply (Folkman, 1986) from the normal host vascular network by the process of angiogenesis (Bergers & Benjamin, 2003). Despite the importance of this neo-vasculature, the system that actually develops is far from adequate. Endothelial cells divide at a slower rate than tumor cells (Denekamp & Hobson, 1982) and thus the developing tumor vasculature is unable to keep pace with the expanding neoplastic cell population. The tumor vasculature formed is also very different from that of normal tissues (Vaupel et al., 1989; Vaupel, 2004). Structurally, it is chaotic, there is a loss of hierarchy, vascular density is abnormal, and the vessels have contour irregularities, are tortuous, dilated, and elongated (Figure 1A). The vessels also are very primitive in nature, having incomplete or missing basement membranes and endothelial lining, and lacking pericytes, smooth muscle, and pharmacological receptors. In addition, there are numerous functional abnormalities, including unstable speed and direction of blood flow, high vascular resistance, increased vascular fragility, red blood cell sludging, leukocyte sticking, and blockage of vessels by circulating white blood cells, platelet aggregates, or tumor cells. All these factors result in the development of areas within the tumor that are characterized by glucose and energy deprivation, high lactate levels and extracellular acidosis, and oxygen deficiency (Vaupel et al., 1989; Vaupel, 2004).
Figure 1.
A. Vascular casts illustrating the differences in vasculature between normal tissues (colon, subcutis, and skeletal muscle) and malignant tumors (colon, melanoma, and sarcoma). Reprinted with permission from Vaupel. (2004). Tumor microenvironmental physiology and its implications for radiation oncology. Seminars in Radiation Oncology, 14, 198–206. B. Illustration of tumor cells growing as a cord around blood vessels from which they obtain oxygen and nutrients. The left side illustrates oxygen diffusion and utilization from the vessel resulting in the development of chronically hypoxic cells at the outer edge of the cord. The right side shows perfusion through the vessel that has been transiently compromised and results in the development of acute hypoxia; examples of the types of flow/oxygen changes reported during a 60 minute period are illustrated in the 4 panels below. Reprinted with permission from Horsman et al. (2012). Imaging hypoxia to improve radiotherapy outcome. Nature Reviews. Clinical Oncology, 9, 674–687.
2.2. Elevated interstitial pressure
The primitive nature of tumor blood vessels results in them being leakier than those of normal tissues. This, coupled with a lack of a functional lymphatic system (Fukumura & Jain, 2007) leads to a significant flow of free fluid in the interstitial space. Consequently, there is a build-up of interstitial fluid pressure (IFP) (Gutmann et al., 1992, Milosevic et al., 2001). Although IFP within tumors may fluctuate (Vaupel, 2011), it is generally uniformly high throughout the center of tumors reaching values of 50–100 mmHg (Vaupel, 2011). However, it drops steeply at the tumor periphery (Boucher et al., 1990), presumably because these areas tend to obtain their blood supply from the normal tissue vessels from which angiogenesis originates.
2.3. Acidic pH
Tumor cells receive essential oxygen and nutrients via diffusion from the vascular supply. But, as these are utilized, gradients are established. For nutrients, especially glucose, these gradients result in energy deprivation at distances from blood vessels (Vaupel et al., 1989). Tumor pH gradients are also found (Helmlinger et al., 1997), with cells distant from vessels being more acidic. Oxygen gradients also occur and regions of low oxygenation (hypoxia) can further influence tumor pH by causing a shift in the balance of cellular energy production towards glycolysis with the subsequent generation of lactate (Vaupel, 2009). Several clinical studies have indeed reported high lactate levels in tumors (Brizel et al., 2001; Walenta & Mueller Klieser, 2004). This results in tumor acidosis, although ATP hydrolysis, glutaminolysis, carbon dioxide production, and bicarbonate depletion, can play important roles (Vaupel, 2009).
2.4. Oxygen deprivation
The microenvironmental parameter that has been the most extensively investigated is hypoxia. Generally, hypoxia is considered to be either chronic or acute in nature (Horsman et al., 2012; Figure 1B). Chronic hypoxia was first suggested by Thomlinson and Gray (1955) and results from a diffusion limitation of oxygen from the blood supply, while acute hypoxia was identified later (Chaplin et al., 1987) and shown to occur due to transient fluctuations in tumor blood flow. This concept of chronic and acute/fluctuating hypoxia is now considered to be an oversimplification (Bayer et al., 2011). Acute hypoxia can result from a total or partial shut-down in perfusion (Kimura et al., 1996); a complete shut-down would starve cells of oxygen and nutrients and result in ischemic hypoxia, which would not be the case for a partial shut-down where plasma flow, thus nutrient supply, can occur. For chronic hypoxia the picture is even more complicated. It can result from a diffusion limitation under “normal” conditions, or be due to reduced oxygen availability as seen in anemic patients or smokers. One also has to consider the level of oxygenation; cells close to the vessel could be slightly hypoxic, while cells next to necrosis could even be anoxic. Using a variety of measurements including microelectrodes, hypoxic markers, and hypoxia-associated molecules (Figure 2), hypoxia has been demonstrated to be a common feature of most solid animal tumor models, human tumor xenografts, and numerous human cancers (Moulder & Rockwell, 1984; Vaupel et al., 1989).
Figure 2.
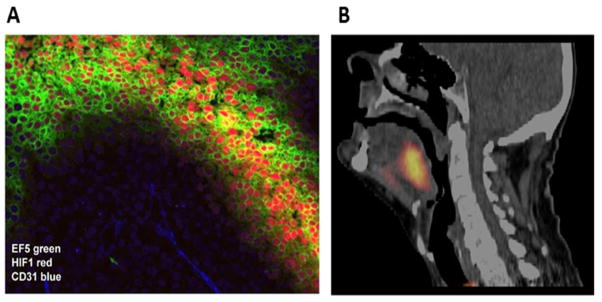
A. Micro-regional distribution of hypoxia in SiHa cervix xenograft illustrating co-localization between the bioreductive nitroimidazole agent EF5 (green) and increased expression of the endogenous hypoxia marker HIF-1 (red) at a distance from blood vessels (CD31, blue). Image courtesy of D. Hedley. B. An example of a patient with a FAZA PET positive oropharyngeal cancer Reprinted with permission from Mortensen et al. (2012). FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiotherapy and Oncology, 105, 14–20.
Hypoxia transcriptionally regulates ~1–2% of all human genes, many of which are driven by the hypoxia-inducible factor 1 (HIF-1); a heterodimeric complex consisting of α and β subunits (Semenza, 2003). In the presence of oxygen HIF-1α is hydroxylated and binds to the von Hippel-Lindau (VHL) ubiquitin ligase, which in turn, targets HIF-1α for proteasomal degradation. In the absence of oxygen HIF-1α is stabilized and forms a heterodimer with HIF-1β to functionally mediate an array of genes involved in angiogenesis, metabolism, survival and invasion such as vascular endothelial growth factor (VEGF), lysyl oxidase (LOX) and matrix metalloproteases (MMPs) (Semenza, 2003). HIF-2α is another hypoxia-regulated transcriptional factor that shares similar sequence and regulatory machinery with HIF-1α (Kaelin, 2008) though its expression appears to be more tissue specific than the ubiquitous HIF-1α (Li et al., 2009). HIF-2α has been shown to promote genes associated with stem cell multipotency (Covello et al., 2006) as well as invasion and angiogenesis (Qing & Simon, 2009). Together, the pleiotropic roles of HIF-α proteins are able to drive tumor cells towards a more aggressive phenotype.
3.0 The impact of the tumor microenvironment on cancer treatment outcomes
3.1. Cancer stem cells and metastases
Cancer stem cells, also known as tumor initiating cells (TICs), represent a subpopulation of tumor cells that selectively possess tumor initiation and self-renewal capacity and the ability to give rise to bulk populations of non-tumorigenic cancer cell progeny through differentiation (Jordan et al., 2006; Gupta et al., 2009). TICs were initially described in leukemias (Lapidot et al., 1994) but subsequently have been identified in a variety of solid cancers (Jordan et al., 2006; Gupta et al., 2009). Although the proportion of such cancer cells in a primary tumor may be small, their existence has been associated with the failure to eradicate primary tumors by conventional anticancer therapies (Baumann et al., 2008) and the spread of human cancers to distant sites (Jordan et al., 2006; Keith & Simon, 2007). Recent studies suggest that TICs have common molecular signatures that are similar to those of pluripotent embryonic stem cells (Ben-Porath et al., 2008; Wong et al., 2008; Bae et al., 2010). Indeed the core transcription factors of this stemness signature, including OCT3/4, SOX2, Nanog, c-Myc and Klf4, have been used to successfully reprogram differentiated somatic cells into pluripotent stem cells (Takahashi et al., 2007). In human embryonic stem cells OCT3/4 works in concert with Nanog and SOX2 to maintain stem cell identity and repress genes that promote differentiation (Wong et al., 2008).
A key step in the progression to malignancy is the transition form an epithelial phenotype to a mesenchymal phenotype, a reprograming known as the epithelial-mesenchymal transition (EMT) (Thiery et al., 2009). Importantly, the activation of the EMT program, which triggers the acquisition of an invasive phenotype, has been shown to be inducible by hypoxia (Zhang et al., 2013). Furthermore, it has been hypothesized that TICs exist in hypoxic regions of tumors. Indeed evidence suggests that hypoxia is able to induce stem-cell like characteristics and embryonic stem cell makers in several cancers (Mathieu et al., 2011; McCord et al., 2009).
Molecular/functional changes initiated by oxygen deprivation impact the non-TIC subpopulation and raises the possibility that hypoxia promotes a transition to the TIC compartment (Figure 3). Recent findings further suggest that hypoxia may play an important role in stem cell maintenance (Keith & Simon, 2007) and that hypoxia regulates TICs by the activation of the HIF-2α transcription factor (Baumann et al., 2008; Li et al., 2009). HIF-2α shares similar sequence and regulatory machinery with HIF-1α. A possible role for HIF-2α in cell stemness is supported by the findings that stem cells exposed to hypoxic conditions demonstrate increased OCT3/4 expression (Keith & Simon, 2007) and that HIF-2α drives several key genes associated with stem cell self-renewal and multipotency, including OCT3/4 and MYC (Fehrer et al., 2007). The role of HIF-2α has been extensively studied in brain tumor models (Baumann et al., 2008; Covello et al., 2006) and results indicate that TICs from gliomas preferentially expressed HIF-2α and multiple HIF-regulated genes when compared to non-stem tumor cells and normal neural progenitors.
Figure 3.
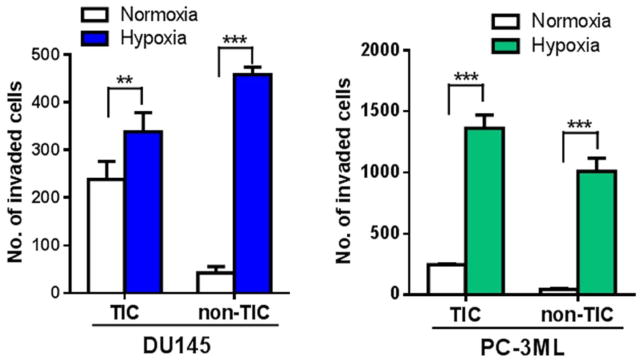
The invasive capacity of both TIC and non-TIC sorted from DU145 and PC-3ML human prostate cancer cells increased significantly upon exposure to hypoxia (1% O2, 24 hours). Columns, mean; bars, SD (n=4); **, p<0.01, ***, p<0.001 (t-test).
In order to establish progressive neoplastic cell growth at a secondary site, disseminating cancer cells must not only have the ability to navigate the metastatic cascade (escape, survival, extravasation) but also display stem cell like characteristics to affect successful colonization (Steeg, 2006). Aberrant primary tumor microenvironments, and the presence of oxygen deficient regions in particular, are now recognized as a key contributors to metastasis (Chaudary & Hill, 2007; Rofstad et al., 2007; Keith & Simon, 2007). Although both acute and chronic hypoxia can exist in solid tumors, their relative impact on the metastatic dissemination of cancer cells may vary (Chaudary & Hill, 2007; Rofstad et al., 2007). While some studies appear to favor chronic hypoxia as the key factor (Algawi et al., 2007), others suggest that acute hypoxia may be equally or more critical in mediating a cancer cell’s metastatic behavior (Rofstad et al., 2007; Cairns et al., 2004; Dai et al., 2011).
Two key signaling controllers, the non-receptor tyrosine kinase c-Src, and the receptor tyrosine kinase c-Met, have been strongly implicated in metastasis (Trusolino et al., 2010; Dai et al., 2012; Siemann et al., 2012). Functionally, these critical signaling molecules modulate multiple cell functions including migration, invasion, survival, and angiogenesis. Both c-Src and c-Met co-activate a variety of signaling cascades that facilitate tumor dissemination such as focal adhesion kinase (FAK), a key enzyme involved in cell adhesion and motility (Schlaepfer & Mitra, 2004). Such metastasis associated signaling pathways may be upregulated by oxygen deprivation. Hypoxia has been shown to promote motility and invasion in tumor cells by inducing c-Met activity and enhancing HGF-stimulated functional behaviors (Pennacchietti et al., 2003; Hara et al., 2006). Since the HGF/c-Met signaling axis upregulates both MET gene expression and HIF-1 activity (Trusolino et al., 2010), amplification of HGF-induced c-Met signaling by hypoxia indicates a positive feedback loop between HIF-1 and c-Met; one that could drive oxygen deprived cancer cells toward a more metastatic state. Oxygen deprivation also may promote c-Src activation (Luis et al., 2007; Mukhopadhyay et al., 1995) and c-Src protein levels have been observed to be higher in chronically hypoxic regions of tumors (Pham et al., 2009). Furthermore, Src-dependent hypoxia-induced VEGF expression may be regulated by HIF-1α (Gray et al., 2005).
Another critical signaling axis in stem cell behavior and metastasis is CXCR4-CXCL12 (Burger et al., 2011). CXCR4 is widely expressed in a variety of neoplastic cell types (Balkwill, 2004) and this axis has been associated with tissue homing of stem cells (Laird et al., 2008). In the tumor microenvironment the CXCR4-CXCL12 axis plays a critical role in tumor cell migration, invasion, adhesion, survival, and the release of angiogenic factors (Petit et al., 2007). Moreover, hypoxia (via HIF-1α) enhances stromal cell CXCL12 secretion and expression of CXCR4 on malignant cells resulting in tumor cell growth stimulation and the recruitment of endothelial cell progenitors (Burger et al., 2011)
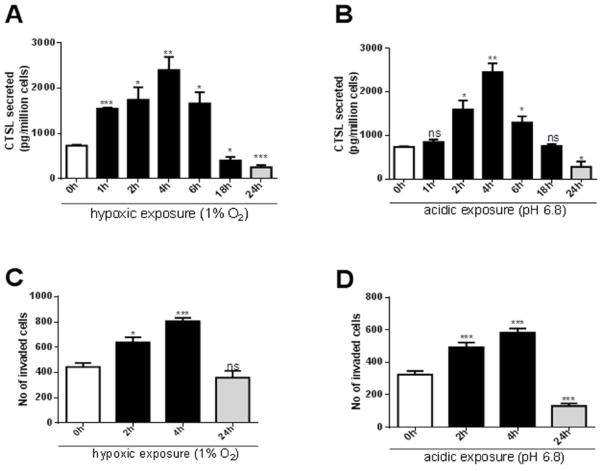
Successful metastasis requires malignant cells to degrade basement membranes and interstitial connective tissue during their escape from the primary tumor as well as their entry into and exit from the bloodstream. This process is greatly facilitated by proteolytic enzymes such as the matrix metalloproteinases (MMPs) and the cysteine protease cathepsins. A family member of the latter, cathepsin L (CTSL), is particularly active in tumor cells and its secretion enhances the metastatic potential of cancer cells through direct proteolysis of components of the extracellular matrix, basement membrane, and E-cadherin (Gocheva et al., 2006). Furthermore, it plays a critical role in the amplification of the proteolytic cascade by activating other key metastasis associated proteases including urokinase plasminogen activator, other cathepsins, as well as certain MMPs (Goretzki et al., 1992; Everts et al., 2006). The over-expression of CTSL occurs in many cancer types (Zajc et al., 2002; Chauhan et al., 1991). It results in aggressive metastatic progression (Chauhan et al., 1991; Gocheva et al., 2006) and has been correlated with clinical outcome (Gocheva et al., 2006; Jagodic et al., 2005). Mechanistically, up-regulation of HIF signaling has been shown to enhance the expression of proteolytic enzymes including CTSL (Jean et al., 2008) and recent findings demonstrate that CTSL secretion is significantly enhanced by acute but not chronic exposures to hypoxia and acidosis (Sudhan & Siemann, 2013). In concert with the enhanced CTSL secretion, brief exposures to hypoxia or acidosis also result in significant enhancement of the metastatic attributes such as migration and invasion (Figure 4).
Figure 4.
A. and B. CTSL secreted levels in PC-3ML cells exposed to hypoxic (1% O2) or acidic conditions (pH 6.8) for the indicated durations followed by reoxygenation or restoration of neutral pH conditions for a total time of 24 h. Secreted CTSL levels were determined by ELISA on cell conditioned media and normalized to cell numbers. Shown are mean and standard error values calculated from three independent experiments. *, p<0.05, **, p<0.005, ***, p<0.001. C. and D. Invasive capacities of PC-3ML cells exposed to hypoxia or acidic pH for indicated durations. Mean and standard error are shown. *, p<0.05, **, p<0.005, ***, p<0.001. Modified from Sudhan et al. (2013). Cathepsin L inhibition by the small molecule KGP94 suppresses tumor microenvironment enhanced metastasis associated cell functions of prostate and breast cancer cells. Clinical & Experimental Metastasis, 30, 891–902.
3.2. Anticancer therapies
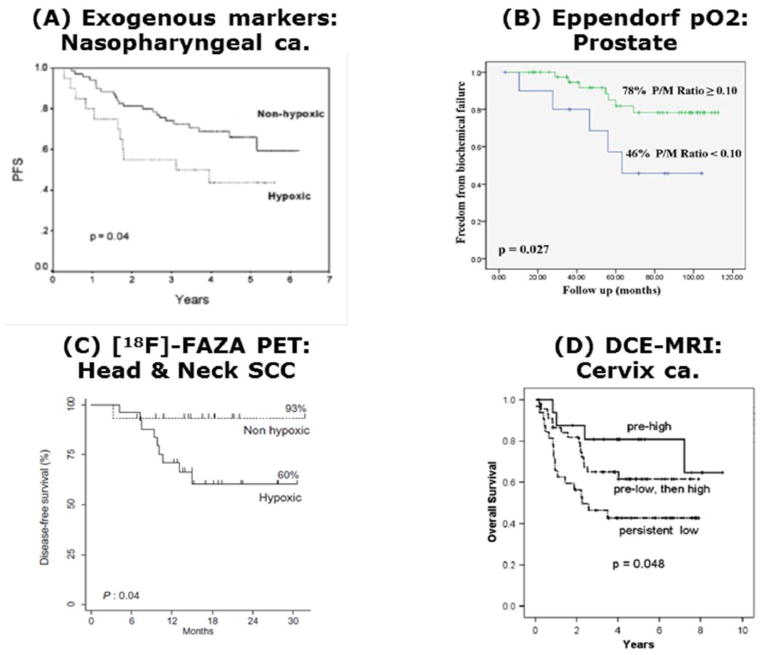
The inadequate vascular supply and adverse microenvironmental conditions play a significant role in influencing tumor response to treatment. This is especially true for hypoxia and radiation. Preclinical in vitro studies going back to the early 1950s (Gray et al., 1953) demonstrated that when oxygen partial pressures were reduced below ~20 mmHg at the time of irradiation, cells became resistant to the radiation; oxygen being required to make the radiation damage irreversible. Numerous in vivo studies that later confirmed the existence of hypoxia in animal and human tumor xenografts, were also able to demonstrate that such hypoxia reduced the efficacy of radiation (Moulder & Rockwell, 1984). Clinical studies have now applied a range of methodologies to demonstrate that hypoxia exists in human tumors and that it can influence radiation response. (Horsman, 1998; Horsman et al., 2012). These techniques include indirect approaches based on estimates of tumor vascularization (Horsman, 1998) and perfusion (Horsman et al., 2012). They also include more direct methods such as the upregulation of endogenous genes/proteins (Rademakers et al., 2008), the binding of exogenous markers to regions of tumor hypoxia (Horsman et al., 2012), or assessment of oxygen partial pressure distributions with polarographic electrodes (Hoeckel et al., 1993; Nordsmark et al., 2005; Brizel et al., 1997; Milosevic et al., 2012). Results of such studies have now shown hypoxia to have a significant influence on both local tumor control and overall survival (Figure 5). For example, in prostate cancer, several studies have correlated hypoxia not only with local recurrence but also early biochemical relapse after radiotherapy, supporting not only the role of hypoxia in radioresistance and increased aggressiveness of the prostate cancer cells, but also suggesting its ability to enhance metastatic potential (Milosevic et al., 2007; Movsas et al., 2002; Milosevic et al., 2012). Importantly, hypoxia or its associated markers have now been identified as independent predictors for disease progression, treatment failures, and greater metastatic potential in cervix, breast, and prostate cancer patients (Hoeckel et al., 1993; Pitson et al., 2001; Chaudary & Hill, 2006; Generali et al., 2006; Stewart et al., 2009 Milosevic et al., 2012). At the molecular level, increased levels of HIF-1α have also been correlated with the risk of metastases in prostate cancer (Kimbro & Simons, 2006) and reductions in disease-free survival in prostate and breast cancer patients (Generali et al., 2006; Kimbro & Simons, 2006). While HIF-2α also has been indicated in prostate malignancy (Brody et al., 2005), its functional role in the metastatic cascade remains unclear.
Figure 5.
Four clinical trials showing the relationship between tumor oxygenation and outcome of therapy. A. Progression free survival (PFS) for 90 patients with nasopharyngeal tumors treated with chemoradiotherapy and stratified for whether their tumors were hypoxic (high expression of HIF-1α and CA IX) or non-hypoxic. Reprinted with permission from Hui et al. (2002). Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clinical Cancer Research, 8, 2595–2604. B. Freedom from biochemical failure for 57 prostate patients treated with brachytherapy in which the prostate/muscle (P/M) mean pO2 ratio estimated using the Eppendorf electrode was above or below 0.10. Reprinted with permission from Turaka et al. (2012). Hypoxic prostate/muscle PO2 ratio predicts for outcome in patients with localized prostate cancer: long-term results. International Journal of Radiation Oncology, Biology, Physics, 82, e433–439. C. Disease-free survival in 40 patients with head & neck squamous cell carcinoma (SCC) based on the pre-radiation therapy estimate of hypoxia as determined by a tumor-to-muscle ratio of > 1.4 from [18F] FAZA PET measurements. Reprinted with permission from Mortensen et al. (2012). FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiotherapy and Oncology, 105, 14–20. D. Overall survival in 98 patients with cervical cancer in which the level of perfusion measured with DCE-MRI was either high pre-radiation, low at pre-radiation and subsequently increasing during therapy, or persistently low. Reprinted from Mayr et al. (2010). Longitudinal changes in tumor perfusion pattern during the radiation therapy course and its clinical impact in cervical cancer. International Journal of Radiation Oncology, Biology, Physics, 77, 502–508.
The pathophysiological characteristics of solid tumors also impact other cancer therapies. This is particularly true for treatments that rely on systemic delivery via the vasculature such as chemotherapy, nano particle therapy, monoclonal antibody based therapy, gene therapy, and immunotherapy. Poorly perfused areas, resulting from low vascular density and fluctuations in blood flow, are known to interfere with the delivery of blood-borne agents (Minchinton & Tannock, 2006).
Extravasation of substances from the vascular supply will also be hindered due to the high IFP (Vaupel, 2011). The characteristics of tumor cells existing under abnormal microenvironmental conditions can further negatively influence the response to anticancer agents; cells distant from the vascular supply will be cycling at a reduced rate and this can provide protection against chemotherapeutic agents with cell cycle based mechanisms of action. Furthermore hypoxic and acidic conditions often co-exist in solid tumors, factors and both are known to directly influence chemotherapeutic agent activity (Vaupel & Osinsky, 2011).
An alternative to conventional cancer therapy is the harnessing of the patient’s own immune response. Unfortunately, tumor cells frequently escape this process of elimination as a result of immunosuppression stimulated by tumor or stromal cell-derived cytokines and growth factors as well as through the modulation of immune cells recruited to the tumor that may aid in promoting tumor cell survival and escape. In fact, in most cases the adaptive immune response against tumor cells is very weak and largely inefficient, since the tumor itself and the surrounding microenvironment down-regulate cytotoxic T lymphocyte (CTL) responses (Mantovani et al., 2008). For example, CTL and natural killer cells have been reported to be inhibited in hypoxic and extracellular adenosine-rich tumor microenvironments, because of immunosuppressive adenosine 3′,5′-monophosphate mediated signaling, triggered by A2A adenosine receptors (Hatfield et al., 2015). Furthermore, secretion of the immune inhibitory molecule programmed cell death ligand-1 (PD-L1) can protect tumor cells from CTL by interaction with programmed death-1 (PD-1), an inhibitory receptor expressed on T and B cells. Hypoxic conditions can significantly contribute to immune modulation (Keith et al., 2007) and upregulation of PD-L1 via HIF-1α can contribute to the tumoral immune escape from CTL (Barsoum et al., 2013).
4.0. The tumor microenvironment as a double edged sword
A dramatic shift in research emphasis arose as a consequence of the recognition that the pathophysiological characteristics which distinguish tumors from normal tissues, aid their progression and dissemination, and make them refractory to conventional anticancer therapies (Figure 6), might be exploitable. Indeed entire new areas of research and approaches to cancer therapy have emerged that seek to take advantage of these features of tumors to turn them into a therapeutic benefit.
Figure 6.
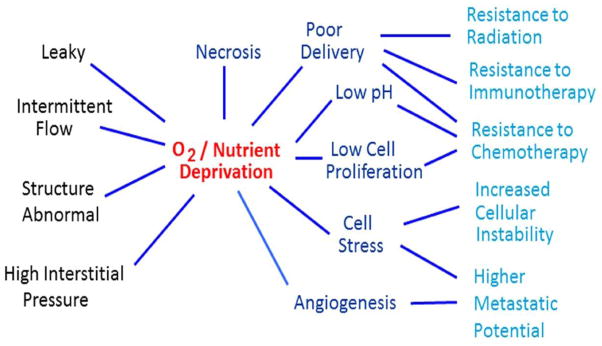
Inherent characteristics of the aberrant tumor blood vessel network result in oxygen and nutrient depravation which negatively impact anticancer therapies and promote tumor cell dissemination resulting in enhanced treatment failures.
Major contributions to this research direction were initially stimulated by the demonstration of greater bioreductive activation of certain chemotherapeutic agents (Satorelli, 1988) and the identification of compounds which not only sensitized hypoxic tumor cells to radiation but also showed significant preferential toxicity to cells lacking oxygen (Brown, 1998). The subsequent recognition that a number of genes, including growth factors, oncogenes, transcriptional factors, and glycolytic enzymes, can be modulated by hypoxia (Giaccia, 1996; Graeber et al., 1996) formed the basis of many current molecular targeting strategies seeking to take advantage of the tumor microenvironment. Simultaneously, vascular targeting approaches emerged to take advantage of the fact that the microvessel networks that develop in tumors are quite unlike the well-defined microvascular architecture of normal tissues, in both structure and function (Siemann & Horsman, 2009). The concept that such critical differences between tumors and normal tissue vasculature may be exploitable remains the central principle of vascular-directed anticancer therapy in the present day.
4.1. Vascular directed therapies
The significance of the tumor neo-vasculature for tumor growth and development makes it an ideal target and two major therapeutic approaches have emerged. One involves inhibiting new vessel formation with angiogenesis inhibitors (AIs), and the other utilizes vascular disrupting agents (VDAs) to damage the already established tumor vessels (Siemann et al., 2000; Horsman & Siemann, 2006; Siemann & Horsman, 2009). In addition to the specific vessel directed therapeutic strategies a number of other anticancer therapeutics can impact the tumor blood vessel network indirectly. In all cases, modification of the tumor vascular supply would also be expected to change the tumor microenvironment.
4.1.1 Angiogenesis inhibitors
For AIs, the effects on the tumor microenvironment are complex and controversial. It has been suggested that targeting angiogenesis could reduce or abolish vascular abnormalities leading to vessel stabilization or normalization, resulting in a more efficient vasculature similar to that seen in normal tissues (Jain, 2001). This would be expected to lead to improved delivery of oxygen and nutrients to the tumor. Indeed, to date some 19 pre-clinical studies have reported that following treatment with a variety of AIs there is a decrease in tumor hypoxia (for review see Horsman & Siemann 2006; Siemann et al., 2014). The AIs have included those targeting VEGF (e.g., bevacizumab, DC101), tyrosine kinase inhibitors (vandetanib, sunitinib, SU5416), and a variety of other agents (e.g., TNP-470, suramin, endostatin, thrombospondin, thalidomide, nucleolin antagonist), with oxygenation measurements made using both direct (e.g., probe techniques, oximetry) and indirect approaches (e.g., hypoxic marker binding, radiation response, gene expression). Typically, the improvement in oxygenation associated with vessel normalization only lasted a few days despite the drug treatment being continued. The controversy arises from the finding that a further 21 pre-clinical studies, which again used hypoxic markers, oxygen probes, radiation response or gene expression techniques to monitor tumor oxygenation, reported that after AI treatment the levels of hypoxia increased. This was true for VEGF targeting agents (e.g., bevacizumab, DC101) tyrosine kinase inhibitors (e.g., sunitinib, pazopanib, AG-013736, PTK787/ZK222584), as well as TNP-470, suramin, anginex, endostatin, and nelfinavir (for review see Horsman & Siemann 2006; Siemann et al., 2014). Furthermore, 9 other studies using the same AIs (e.g., bevacizumab, DC101, sunitinib, SU5416, endostain) plus other inhibitors (e.g., sorafanib, axitinib, arginine deiminase) found no changed in tumor oxygenation using the same direct and indirect measures. It could be argued that the differential effects on tumor oxygenation following AI treatment were the result of different drug doses, treatment schedules, or time of oxygenation measurement. While this is likely the case, further complicating matters, two independent studies with DC101 using the same treatment, and assessment technique and schedule, reported both an improved oxygenation (Winkler et al., 2004) and increased hypoxia (Fenton et al., 2004), implying that the physiologic effects of AI treatments may also be strongly tumor-dependent. The lack of a uniform response on tumor oxygenation and in particular the possibility that AI therapy may induce hypoxia, a known enhancer of tumor cell dissemination (Cairns et al., 2004; Chaudary & Hill, 2007; Rofstad et al., 2007; Keith & Simon, 2007), may support concerns that such therapy could increase metastasis (Paez-Ribes et al., 2009; Ebos et al., 2009).
Another major pathophysiological effect of AIs that has often been reported involves changes in IFP. Following treatment with AIs, IFP drops (Tong et al., 2004). With decreasing vascular density, the most likely explanation for a decrease in IFP would be a decrease in the number of tumor cells, and indeed, there is evidence that treatment with AIs can lead to tumor cell killing (Teicher et al., 1994; Gong et al., 2003). However, other mechanisms may also be involved. One study with the tyrosine kinase inhibitor PTK787/ZK 222584 showed using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in a murine renal cell carcinoma that this inhibitor could decrease vessel permeability and such an effect would also be expected to reduce IFP (Drevs et al., 2002).
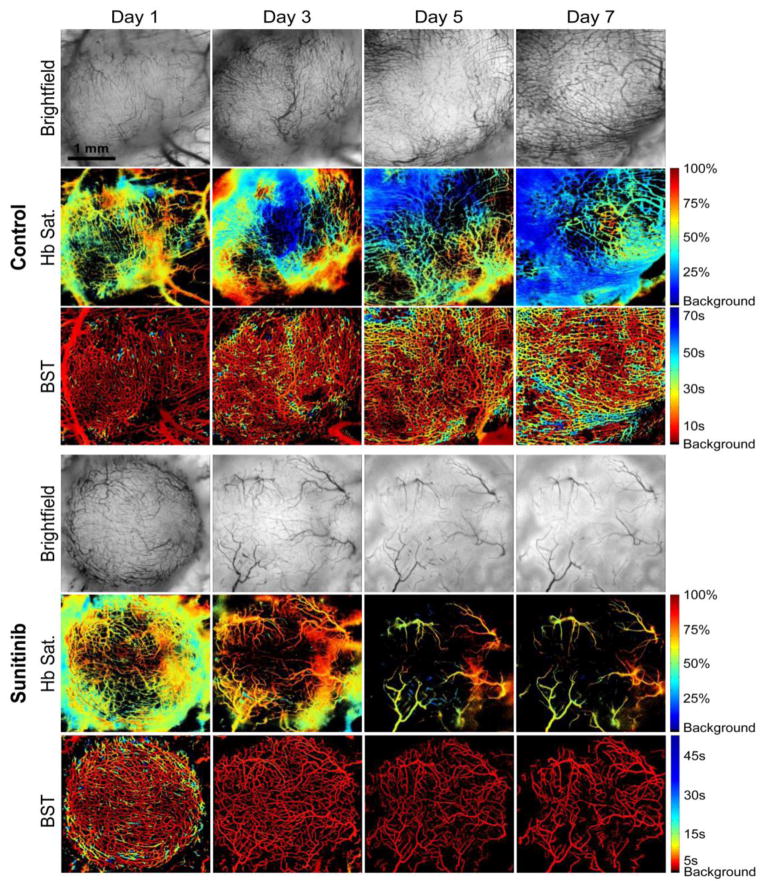
Despite the controversies surrounding their pathophysiologic effects, there is little doubt that AIs can have a significant impact on the tumor blood vessel network (Figure 7) and abundant evidence exists that their judicious application can improve the response to conventional anticancer therapies (Horsman & Siemann, 2006; Siemann & Horsman, 2009). Moreover, the recent reports that improvements in outcomes with AI therapy may be correlated with increased tumor perfusion and oxygenation (Batchelor et al., 2013; Jain, 2014) are encouraging. As is the suggestion that low dose AI administration may yield preferential improvements in these physiologic parameters (Reynolds et al., 2009; Wong et al., 2015; Jain, 2014) but the generality of this observation across agent, dosing schedule, and tumor type remains to be established.
Figure 7.
Bright field images and corresponding hemoglobin saturation and blood supply time (BST) maps of an untreated Caki-2 renal cell tumor and a tumor receiving daily administration of Sunitinib (oral gavage, 100 mg/kg) over time. Treatment with Sunitinib resulted in an inhibition of tumor vessel development and higher microvessel oxygenation values in comparison to controls. In addition, treatment with Sunitinib resulted in faster BST values in comparison to controls, indicating the formation of a more organized vascular network. Modified from Lee et al. (2014). In vivo spectral and fluorescence microscopy comparison of microvascular function after treatment with OXi4503, Sunitinib and their combination in Caki-2 tumors. Biomedical Optics Express, 5, 1965–1979.
4.1.2. Vascular disrupting agents
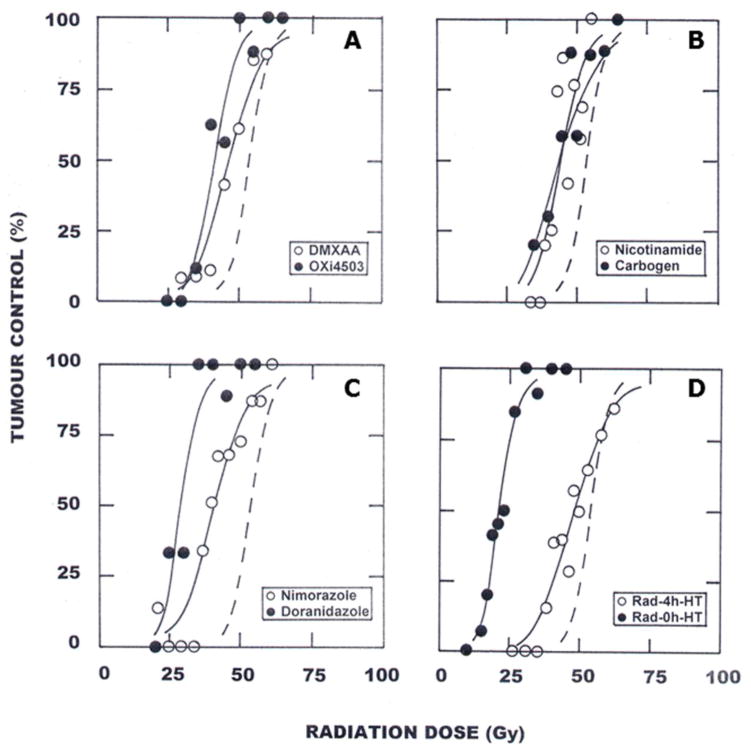
In contrast to the effects seen with AIs, the changes in tumor oxygenation induced by VDAs are less contentious. As a consequence of VDA induced vascular damage, tumor blood perfusion is inhibited, resulting in a reduced delivery of oxygen and nutrients (Horsman & Siemann, 2006). Direct measurements of tumor oxygenation after treatment with a variety of VDAs confirm this (Zhao et al., 2005; Iversen et al., 2013). VDA-induced reductions in functional tumor vasculature are also reflected in other microenvironmental changes including significant and rapid decreases in both extracellular (Sakaguchi et al., 1992) and intracellular pH (Breidahl et al., 2006). IFP also changes, but here the effects can be divergent with both increases and decreases having been observed. Measurements of IFP, primarily made using the wick-in-needle technique, generally show a decrease in IFP after treatment with VDAs (Skliarenko et al., 2006; Nielsen et al., 2010). But other studies have reported no change (Eikesdal et al., 2002), increased (Eikesdal et al., 2002), or fluctuating IFP (Skliarenko et al., 2006) following VDA exposure. There is, however, general consensus that the VDA treatment induced collapse of existing tumor vasculature results in secondary tumor cell death and extensive centrally situated tumor necrosis (reviewed by Siemann, 2011). Thus VDAs may be complementary to radiotherapy and chemotherapy treatments because they eliminate aberrant microenvironments in regions of the tumor core. Indeed, there is now good pre-clinical evidence that significant improvements in tumor response following treatment with radiation or chemotherapy can be improved by combining these conventional treatments with a variety of VDAs (Horsman & Siemann, 2007; Siemann & Horsman, 2009). This is illustrated for radiation in Figure 8A using representative agents from the two major groups of VDAs, namely tubulin binding agents (e.g., OXi4503) and flavanoid compounds (e.g., DMXAA). Limited studies combining VDAs with radiation in normal tissues have reported no additional effect, thus the enhancements seen are tumor specific (Horsman & Siemann, 2007).
Figure 8.
C3H mammary carcinomas in CDF1 mice were locally irradiated with single doses of radiation and the percentage of animals showing tumor control within 90 days were recorded. The radiation dose-response curve, determined from logit analysis, is shown as the dashed line in each figure. Each figure also shows how this radiation response curve is modified by various treatments. A. within 1-hour after irradiating i.p. injecting 50 mg/kg OXi4503 (●) or 20 mg/kg DMXAA (○); B. carbogen breathing for 5 minutes before and during irradiation (●) or an intraperitoneal (i.p.) injection with 1000 mg/kg nicotinamide 30 minutes before irradiating (○); C. i.p. injecting 500 mg/kg nimorazole (○) or doranidazole (●) 30 minutes prior to irradiating; D. irradiating (Rad) tumors in the middle (0h) of heating (HT) at 42.5°C for 60 minutes (●) or irradiating (Rad) tumors and heating (HT) at 42.5°C for 60 minutes 4-hours (4h) later (○). Modified from Horsman et al. (2011). Impact on radiotherapy. In D. W. Siemann (Ed.), Tumor Microenvironment (pp. 353–376). Chichester: John Wiley & Sons, Ltd.
Given their disparate modes of action, a double assault on the tumor vasculature through the combined application of AIs and VDAs can lead to complimentary and markedly enhanced treatment outcomes in preclinical investigations (Siemann & Shi, 2004; 2008) and clinical assessments of this concept are in progress (Monk et al., 2014). Combining both AIs and VDAs with more conventional therapies would also likely result in significant improvements in response.
4.1.3 Indirect modifiers of tumor vasculature
In addition to therapeutic strategies designed to directly target the tumor blood vessel network, many other agents currently in use for cancer management can affect components of the angiogenesis network or inhibit angiogenesis indirectly (Ferrara & Kerbel, 2005). These include a variety of signaling inhibition strategies as well as conventional chemotherapeutic agents. With regard to the former, trastuzumab, a monoclonal antibody that interferes with the HER2/neu receptor, also has been reported to inhibit VEGF expression (Petit et al., 1997). Similarly, the small molecule Src targeting agents saracatanib and dasatinib significantly reduce tumor angiogenesis both in vitro and in vivo (Siemann et al., 2012; Rice et al., 2012). Interestingly, protolytic enzymes long recognized as major contributors to the dissemination of tumor cells not only can elicit pro-angiogenic endothelial cell function but their selective targeting can impair tumor associated angiogenesis (Sudhan et al., 2015). Other examples abound but are beyond the scope of the present review. With regard to chemotherapeutic agents, it is their use in a metronomic fashion, defined as a chemotherapy treatment that is given more often but in lower doses than conventional chemotherapy, that has received the most attention (Kerbel & Kamen, 2004). Initially investigated because of the less severe adverse effects associated with conventional anticancer drug therapy (Romiti et al., 2013), it is now known that low dose metronomic chemotherapy regimen can elicit significant effects in the developing tumor vasculature. Indeed studies in preclinical tumor models have shown that metronomic treatment with chemotherapeutic agents such as doxorubicin and cyclophosphamide can impact vascular density and endothelial cell survival compared to conventional chemotherapy treated mice (Zhou et al, 2012; Digklia et al., 2014). Even greater effects on tumor associated angiogenesis are noted when combined metronomic chemotherapy is combined with VEGF pathway targeting drugs (Zhou et al, 2012; Digklia et al., 2014). Taken together, findings such as these have led to the initiation of clinical investigations evaluating metronomic chemotherapy in a number of disease settings and the suggestion that such a treatment strategy in conjunction with AI therapy ought to be given clinical consideration (reviewed in Torimura et al., 2013; Digklia et al., 2014). Still, how such treatments impact the tumor microenvironment and the possible therapeutic ramifications remains poorly understood.
4.2. Modifiers of oxygen delivery
One of the earliest approaches applied to try and improve the microenvironmental conditions within tumors was to simply increase oxygen supply. Experimental studies reported that by allowing mice to breathe either oxygen or carbogen (95% oxygen + 5% carbon dioxide), before and during irradiation, significantly enhanced the response of murine tumors to radiation (Du Sault, 1963; Suit et al., 1972). The best effect was generally seen when the gasses were inspired under hyperbaric (typically 3 atmospheres) rather than normobaric conditions (Du Sault, 1963; Suit et al., 1972), which is perhaps not surprising because hyperbaric conditions would be expected to saturate the blood with oxygen more than normobaric conditions. However, later studies indicated that the radiosensitizations produced by normabaric oxygen or carbogen (Figure 8B) are in fact quite substantial (Siemann et al., 1977; Rojas, 1991; Grau et al., 1992). Clinical trials with hyperbaric oxygen (HBO) were also initiated early (Churchill-Davidson, 1968). Although benefit was not always obtained, significant improvements in local tumor control and subsequent survival were certainly seen in the large multicenter clinical trials conducted by the UK Medical Research Council for both uterine cervix and advanced head and neck cancer (Dische, 1978; Overgaard, 1989).
Breathing normobaric high oxygen content gas has also been tried clinically but the early studies failed to show any dramatic improvement in radiation response (Bergsjø & Kolstad, 1968; Rubin et al., 1979). This may have been due to the failure to achieve the optimum pre-irradiation gas breathing time. A number of experimental studies have shown this to be critical for the enhancement of radiation damage, and that it can vary from tumor to tumor (Suit et al., 1972; Siemann et al., 1977; Rojas, 1991; Chaplin et al., 1993). More recent studies with carbogen in head and neck cancer patients, using a short pre-irradiation breathing time, have yielded conflicting findings, with both a benefit (Kaanders et al., 2002) and no improvement (Mendenhall et al., 2005) in radiation response observed. The positive results in the former study may have been because carbogen was administered as part of the ARCON (Accelerated Radiation, CarbOgen, and Nicotinamide) therapy; accelerated radiotherapy was included to address the issue of accelerated repopulation that is known to occur after irradiation (Petersen et al., 2001), and nicotinamide was added because this agent can prevent the fluctuations in blood flow that ultimately lead to acute hypoxia (Horsman, 1995). The ability of nicotinamide to enhance tumor radiation response is shown in Figure 8B. More recent results from the ARCON study found a small improvement in radiation response in head & neck cancer patients; an effect that was larger when patients were stratified into having hypoxic or non-hypoxic tumors (Janssen et al., 2012). A slightly better response was reported in a BCON trial (Hoskin et al., 2010) in which bladder cancer patients receiving radiation therapy were also treated with carbogen and nicotinamide.
While the inspiration of high oxygen gases to overcome tumor hypoxia has been predominantly applied in investigations seeking to enhance the treatment efficacy of radiotherapy, recent evidence suggests that such a strategy may have utility in other forms of cancer therapy. In particular, preclinical investigations have shown that improved oxygenation may overcome hypoxia-adenosinergic immunosuppression leading to enhanced antitumor responses and reductions in metastasis (Hatfield et al., 2015). These encouraging findings suggest that reducing tumor hypoxia through oxygen inspiration may also have utility as an adjuvant to cancer immunotherapy.
The transport of oxygen to tissues in the body is via hemoglobin in the blood. Thus, it is not surprising that low hemoglobin concentration has, in general, a negative impact on tumor radiation response (Grau & Overgaard, 1998). In anemic patients, hemoglobin concentration can be increased via blood transfusion (Thomas, 2002) or by stimulation with the hormone erythropoietin (Hoskin et al., 2009). However, conflicting results have been reported for the ability of transfusion to improve radiation response, with both benefits (Evans & Bergsjø, 1965; Grogan et al., 1999) and no effect (Overgaard et al., 1998) reported. With erythropoietin (EPO) the situation is even more complicated in that patients receiving EPO had a poorer prognosis after radiation therapy than those patients that did not receive EPO (Hoff 2012).
Other approaches suggested to improve oxygen delivery to tumors include the use of artificial blood substances, such as perfluorochemical emulsions (PFE), which are small particles capable of carrying more oxygen than hemoglobin (Rockwell, 1985); modifiers of the hemoglobin-oxygen (Hb-O2) dissociation curve, which improves the oxygen offloading capacity of blood (Siemann & Maclear, 1986; Hirst & Wood, 1991); or inhibiting cellular oxygen consumption, thereby increasing the diffusion distance of oxygen (Zannella et al., 2013). While PFEs and Hb-O2 modifiers worked well in pre-clinical studies, they never reached clinical evaluation. The lead agent for the oxygen consumption modifiers is metformin, which has undergone extensive clinical use for the treatment of diabetes and has been linked to decreased rates of certain types of cancer (Zhang et al., 2013). However, the limited pre-clinical studies specifically attempting to demonstrate the potential of metformin to decrease cellular oxygen consumption and subsequently reduce tumor hypoxia have involved using clinically unrealistic drug doses (Zannella et al., 2013), so the clinical relevance of this approach remains questionable.
4.3. Radiation sensitizers
Probably the most extensively investigated method for overcoming tumor hypoxic cell radioresistance is the use of chemical agents that mimic oxygen. In the early 1960s it was found that the efficiency of radiosensitization was directly related to electron-affinity (Adams & Cooke, 1969) and that ultimately led to in vitro studies demonstrating preferential radiosensitization of hypoxic cells by highly electron-affinic nitroaromatic compounds (Adams et al., 1976). The initial compounds were nitrobenzenes, followed by nitrofurans, and then nitroimidazoles, with the latter compounds subsequently shown to be effective at enhancing radiation damage in tumors (Overgaard, 1994; Figure 8C); unlike oxygen, these drugs are not rapidly metabolized by the tumor cells through which they diffuse and thus can penetrate farther than oxygen and so reach all cells in the tumor.
The first agent chosen for clinical evaluation was metronidazole in brain tumors (Urtasun et al., 1976). It was soon replaced by misonidazole and a large number of clinical trials were undertaken (Overgaard, 1989; Overgaard, 1994). Unfortunately, most misonidazole trials were unable to generate significant improvement in radiation response, although a benefit was seen in some trials, particularly the Danish Head & Neck Cancer (DAHANCA 2) study (Overgaard et al., 1989). The general lack of benefit has been attributed to clinical evaluation in tumor sites lacking convincing evidence of the existence of hypoxia, the small number of patients in many of the trials, and because the drug doses necessary for effective radiosensitization also produced substantial dose-limiting clinical toxicity. Further clinical studies focussed on identifying more efficient or less toxic hypoxic sensitizers. The first of these was a European trial with pimonida-zole in uterine cervical cancer, but the preliminary results were disappointing (Dische et al., 1993). Etanidazole was then tested in two other multicenter trials in head and neck cancer, but the results showed no benefit (Lee et al., 1995; Eschwege et al., 1997). Additional studies with nimorazole, a less efficient sensitizer but less toxic drug, in head and neck cancer patients (DAHANCA 5) showed a highly significant benefit in terms of improved loco-regional tumor control and disease-free survival (Overgaard et al., 1998). A more recent International Atomic Energy Agency (IAEA) trial with the 3-nitrotriazole compound sanazole (AK-2123) in uterine cervical cancer also demonstrated a significant improvement in both local tumor control and overall survival (Dobrowsky et al., 2007), while a Japanese randomised trial with the 2-nitroimidazole doranidazole (PR-350) in locally advanced pancreatic cancer reported a significant increase in long term survival (Karasawa et al., 2008). The positive clinical results with the nitroimidazoles support the general consensus that if a non-toxic hypoxic modification can be applied then such treatments may certainly be relevant as a baseline therapy together with radiotherapy for certain types of cancer. However, the only agent that has been incorporated into standard radiotherapy treatment is nimorazole in head and neck cancer; a treatment strategy currently employed only in Denmark.
More recent studies have suggested another hypoxia related approach that may sensitize tumor cells to radiation and even certain types of chemotherapy. This is based on the findings that hypoxia decreases DNA repair (Bristow & Hill, 2008; Chan & Bristow, 2010). The repair pathways affected include those responsible for DNA mismatch repair, nucleotide excision repair, and double strand break repair; the latter includes both homologous recombination and to a lesser extent nonhomologous end-joining. It has been suggested that hypoxic cells showing such repair deficiencies would be more sensitive to both chemotherapeutic agents (e.g., alkylating agents and topoisomerase inhibitors) and radiation (Bristow & Hill, 2008; Chan & Bristow, 2010). There is also evidence that cells with defects in homologous recombination pathways can be preferentially sensitized to inhibitors of poly-ADP ribose polymerase 1, while deficiency in various mismatch repair proteins have been shown to be influenced by DNA polymerase inhibitors (Chan & Bristow, 2010). Clearly this is a new area that could have potential clinical application and further studies are warranted.
4.4. Bioreductive drugs
An alternative strategy to overcome the problem of hypoxic tumor cells is to selectively kill them by using hypoxic cell cytotoxins. Because hypoxia is essentially unique to tumors, such agents would offer significant therapeutic potential (i.e., preferentially killing hypoxic cancer cells that are resistant to conventional anticancer therapy while sparing aerobic normal tissues). Bioreductive or hypoxia-activated prodrugs are typically non-toxic compounds that are reduced by enzyme(s) in hypoxic tumor cells. The enhanced activities of reducing enzymes in hypoxic regions of solid tumors, such as the nitroreductases, provide means for site-specific conversion of prodrugs to yield the cytotoxic products. Selectivity for hypoxia is usually a result of a futile redox cycling in which oxygen is able to reverse or inhibit reduction to the one-electron radial intermediate thereby regenerating the non-toxic parent drug (Rauth et al. 1984; Ahn & Brown, 2007; Wilson & Hay, 2011). Four classes of bioreductive compounds, quinones, nitroaromatics, aliphatic and heteroaromatic N-oxides, have been developed. These have been extensively reviewed (McKeown et al., 2007, Ahn & Brown, 2007; Wilson & Hay, 2011; Guise et al., 2014). All require hypoxic conditions and the presence of specific reductases for activation.
In the early 1980s the natural product mitomycin C was recognized to possess bioreductive activity (Rockwell, 1986). Although the enhancement of hypoxic relative to aerobic cell killing (hypoxic cytotoxic ratio, HCR) for mitomycin C was quite small (<5-fold) (Rockwell, 1986; Cowen et al., 2003), the prototypic bioreductive agent led to the subsequent development of a series of indolequinones. The most promising of these, EO9, showed significant superiority (5-fold greater HCR) to mitomycin C (Saunders et al., 2000). A major driving force behind the development of bioreductive prodrugs was the original recognition that nitroimidazole oxygen-mimetic radiosensitizers such as misonidazole also were metabolized to cytotoxic products selectively in hypoxic tumor cells (Rauth et al., 1984). However, these nitroaromatic hypoxic cell radiosensitizers were only weakly cytotoxic, having only moderate (about ten-fold) selective toxicity for hypoxic cells over oxygenated cells (Sutherland et al., 1982). Subsequent drug development around the nitroimidazole platform led to the first agent, RSU1069, with marked enhancement in hypoxia selectivity (Stratford et al., 1986). Two other promising nitro compounds are the bioreductive prodrugs PR-104 and TH-302. The former gives rise upon nitro reduction to cytotoxic metabolites which result in DNA interstrand cross-links (Guise et al., 2012). The latter, a 2-nitroimidazole-based nitrogen mustard prodrug, releases bromoisophosphoramide mustard (Duan et al., 2008) and shows significant selectivity for hypoxic cells (Meng et al., 2012). The most advanced of the aromatic N-oxides class of bioreductive agents is tirapazamine (Ahn & Brown, 2007). This agent has shown HCR values of up to 200 in murine and 50 in human cell lines (Zeman et al., 1986). Another group of compounds that has shown potential as bioreductive drugs are the aliphatic N-oxides. The agent which best represents this class is the bis-N-oxide banoxantrone AQ4N (Patterson & McKeown, 2000). AQ4N is reduced under hypoxic conditions to yield the cytotoxic product AQ4 which has high DNA binding affinity and also acts as a topoisomerase II inhibitor (Patterson & McKeown, 2000).
Although some have shown in vivo activity on their own, bioreductive prodrugs are ideally combined with therapies that target relatively well oxygenated tumor cells such as ionizing radiation and chemotherapeutic agents, because their modes of action will be complementary (i.e., bioreductive prodrugs have the potential to eliminate the hypoxic tumor cells that are resistant to the conventional therapies). Indeed in vivo studies demonstrate that bioreductive agents such as RSU1069, tirapazamine, and AQ4N, could enhance the response to single and fractionated dose radiotherapy irrespective of whether the drug was given before or after radiotherapy (McKeown et al., 1995; Stratford et al., 2003). Furthermore, preclinical in vivo combination studies with AQ4N, tirapazamine, and nitroheterocyclics also have shown that these agents can enhance the anti-tumor effects of conventional anticancer drugs, most notably alkylating chemotherapy (Patterson & McKeown, 2000; Siemann & Mulcahy, 1986; Siemann, 1995). Similarly, PR-104 has recently been reported to enhance the efficacy of several established anticancer drugs, including docetaxel and gemcitabine (Patterson et al., 2007). Importantly preclinical investigations suggest that potential bioreductive agent therapy benefits may not be confined to primary tumors but also impact the control of metastatic disease (Siemann & Alliet, 1987; Lunt et al., 2005).
While the presence of hypoxia is typically considered to be a detrimental factor in the curability of tumors, this property of a tumor may be exploitable in therapies designed specifically to attack the hypoxic cell subpopulation. The fact that very low levels of oxygen are unique to solid tumors provides an excellent basis for tumor selectivity which continues to drive the preclinical development and clinical evaluation of this therapeutic approach (Table 1; Wilson & Hay, 2011).
Table 1.
Bioreductive prodrugs of DNA-reactive cytotoxins recently or currently in clinical development (modified from Wilson & Hay, 2011)
| Prodrug | Current clinical status | Company or institution | Chemical class | Mechanism of cytotoxicity |
|---|---|---|---|---|
| Tirapazamine (SR 4233) | Phase III, cervix (closed) | SRI International/NCI | Aromatic N-oxide | Complex DNA damage |
| Apaziquone (E09) | Phase III, bladder (closed) | Spectrum | Quinone | ICL |
| TH-302 | Phase I/II, multiple (active) | Threshold | Nitro | ICL |
| PR-104 | Phase I/II, leukaemia (active) | Proacta and University of Auckland | Nitro | ICL |
| Banoxantrone (AQ4N) | Recent Phase I/II | Novacea | Aliphatic N-oxide | TOPOII |
| Caricotamide (EP-0152R) plus tretazicar (CB1954) | Phase II, HCC (discontinued) | BTG | Nitro | ICL |
| RH1 | Recent Phase I | CRUK | Quinone | ICL |
| NLCQ-1 | Preclinical | Evanston Hospital | Nitro | TOPOII or multiple? |
| SN30000 (CEN-209) | Preclinical | Centella and University of Auckland | Aromatic N-oxide | Complex DNA damage |
| SN29730 | Preclinical | University of Auckland | Nitro | Adenine N3 alkylation |
| KS119W | Preclinical | Yale University | Nitro | Guanine O6 ICL |
CRUK, Cancer Research UK; HCC, hepatocellular carcinoma; ICL, DNA interstrand crosslink; NCI, US National Cancer Institute; TOPOII, topoisomerase II
Used with permission from Wilson et al (2011) Targeting hypoxia in cancer therapy. Nature Reviews. Cancer, 11, 393–410.
4.5. Hyperthermia
Another, less conventional yet clinical applied therapy, in which the poor pathophysiological characteristics of tumors is actually beneficial, is hyperthermia. Blood flow is one of the major mechanisms by which heat is dissipated from tissues, and this will affect the ability to heat tumors (Horsman et al., 2006); the poorer the blood supply the easier it should be to heat. This has been demonstrated in vivo when blood flow was compromised using agents that could reduce tumor blood flow (Voorhees & Babbs, 1982; Horsman et al., 1989). In vitro studies have also reported cells under hypoxic conditions to be more sensitive to killing by hyperthermia than cells in well oxygenated environments (Overgaard & Bichel, 1977; Gerweck et al., 1979). However, this is not a consequence of hypoxia per se because under well-defined nutrient conditions, acute hypoxia does not significantly alter cellular response to heat (Gerweck et al., 1979). However, cells under prolonged oxygen deprivation will show an increased sensitivity to heat, an effect that is the result of chronically hypoxic cells becoming acidic (Overgaard & Bichel, 1977). The significance of pH in influencing response to hyperthermia has led to attempts to modify tumor pH and thereby increase efficacy. This can be achieved by administration of glucose (Urano, 1994) and the potential of this approach has been shown to improve heat response (Hiroaka & Hahn, 1990). Interestingly, such an approach can enhance the anti-tumor effect of certain chemotherapy agents when used alone or combined with hyperthermia (Urano, 1994).
Furthermore, numerous preclinical studies have shown both in vitro and in vivo (for review see Horsman & Overgaard, 2007) that irradiating tumors and heating with temperatures up to 43°C at or near the same time substantially enhanced radiation response (Figure 8D). The exact mechanism by which heat sensitizes cells to radiation is not known, but most evidence suggests that heat primarily interferes with the cells ability to cope with radiation-induced DNA damage (Kampinga & Dikomey, 2001; Roti Roti, 2004). Because this will occur in both tumors and normal tissues, a therapeutic benefit will only be obtained if tumors can be preferentially heated. Furthermore, when a time interval is introduced between irradiating and applying heat, the degree of enhancement is reduced (Figure 8D), reaching a plateau with an interval of 2–4 hours depending on the tumor type and the heat treatment applied (Horsman & Overgaard, 2007). In this situation, the enhancement that remains is believed to be simply a result of the heat killing the radiation resistant hypoxic cell population, and since normal tissues do not contain hypoxia the effect is tumor specific and results in a clear therapeutic benefit (Horsman & Overgaard, 2007). In the clinic, a meta-analysis of all published trials (1861 patients from 23 trials), in which patients were randomized to radiation or radiation plus heat, demonstrated significant improvements in local tumor control, the most relevant endpoint for such locally applied treatments, in a number of distinct cancer sites including chest wall, cervix, rectum, bladder, melanoma, and head and neck (Horsman & Overgaard, 2007). In some of these clinical studies improvements in overall survival also were observed (Horsman & Overgaard, 2007). Despite these positive clinical results, hyperthermia has not become an established adjuvant for radiation therapy. This is partly because of the difficulty in achieving and maintaining adequate tumor heating, especially in deep seated tumors, but is also due to the need to establish equipment and personnel dedicated to the application of hyperthermia.
4.6. Exercise
As described in the previous sections, a variety of strategies have been undertaken to diminish or eliminate tumor hypoxia. Although these approaches showed merit and a meta-analysis confirmed the efficacy of hypoxia-directed treatments, outcomes have been largely disappointing for reasons that include failure to preselect patients with hypoxic tumors, physiologic counter responses to high oxygen content gases, or dose-limiting side effects of hypoxic sensitizers and bioreductive agents. One approach that has not been investigated is the application of exercise to alter whole body hemodynamics in order to increase tumor oxygen delivery and decrease tumor hypoxia.
Exercise elicits many health benefits (Centers for Disease Control and Prevention, 2013) including improvements in aerobic capacity, lowered body fat, reduced depression, and increased immune function. With respect to the latter, evidence is accumulating that physical exercise can modulate the cells of the immune system to have positive effects on their function and response to pathogens (Pedersen & Hoffman-Goetz, 2000; Cheng et al., 2013; Gholamnezhad et al., 2014). Exercise investigations in cancer have focused primarily either on cancer development/prevention or its application to ameliorate complications and wellness in cancer survivors. The idea that exercise may prevent cancer development was first postulated early in the 20th century (Cherry, 1922). Indeed, several investigators have reported that cancer mortality (all sites) was inversely related to the amount of exercise/physical activity performed (Garfinkel & Stellman, 1988; Paffenbarger et al., 1986), though with respect to particular cancer types, the effects of exercise on cancer incidence often are equivocal. As to the cancer patient or survivor, the adoption of appropriate exercise regimens appears even more important. For example, studies on breast and prostate cancer patients have shown that exercise performed during the course of radiation therapy decreases treatment related side effects, rendering the therapy more tolerable (Hwang et al., 2008; Windsor et al., 2004; Mustian et al., 2009). Similar modulations of chemotherapy side effects have also been reported (van Waart et al., 2010; Brown et al., 2012). Indeed, the National Cancer Institute has recently provided a bulletin to urge exercise for cancer patients and survivors (Phillips et al., 2012; Schmitz et al., 2010). Notwithstanding this recommendation and despite the fact that physical activity is a fundamental component of our daily life, surprisingly little is known about the effects of either a single bout or a sustained exercise program on the tumor microenvironment or the responses to conventional anticancer therapies such as radiation and chemotherapy. Yet an understanding of how exercise affects tumor perfusion, blood flow, and hypoxia may offer unique opportunities to enhance cancer treatment outcomes and improve optimal cancer patient care.
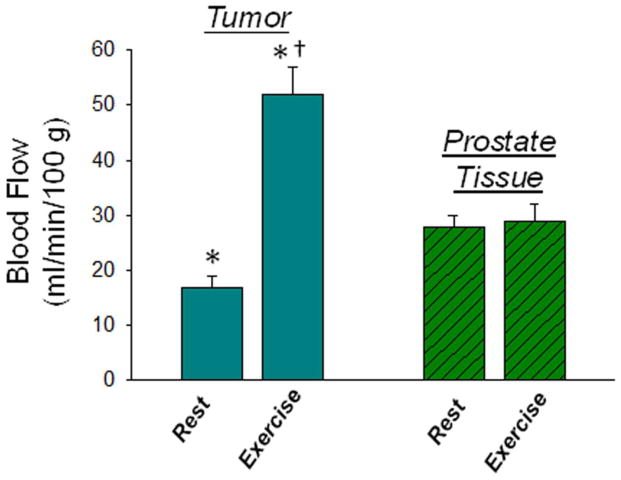
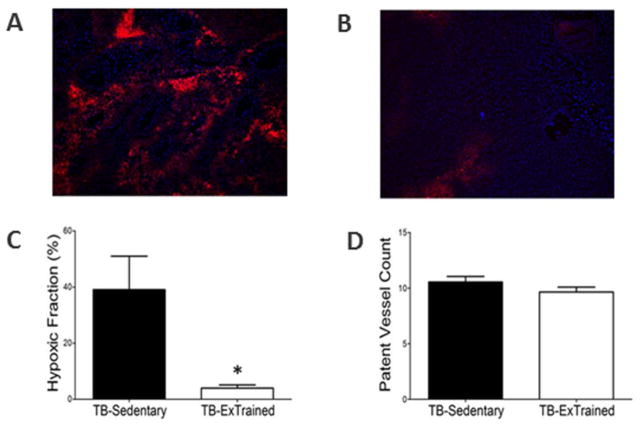
Existing data concerning the effect of exercise on established tumor growth and perfusion in rodent tumor models remains controversial; appearing to be particularly influenced by the choice of tumor implantation site in preclinical investigations. Since rodent skin and immediate subcutaneous tissue has a very low blood flow (Armstrong & Laughlin, 1985), it is possible that subcutaneously transplanted tumors may actually become hypoxic during exercise. However, studies in which tumors were grown at the site of origin (orthotopic implantation) have reported that low intensity exercise does not affect tumor growth (Jones et al., 2012) and reduces the incidence of metastasis (Cohen et al., 1992). These latter findings are consistent recent observations which showed no evidence of increased metastasis or enhanced primary tumor growth in exercised animals bearing orthotopically implanted prostate tumors (McCullough et al., 2013). Furthermore, these studies demonstrated that exercise increased prostate tumor blood flow (>200%) in the absence of blood flow changes in normal prostate tissue (Figure 9); a result due at least in part to an inability of the tumor vasculature to vasoconstrict (McCullough et al., 2014). Importantly the increase in tumor blood flow resulted in a significant reduction in tumor hypoxia (Figure 10). The ability to modulate tumor hypoxia by exploiting the systemic effects of exercise and the dysfunctional vasculature of solid tumors have recently been duplicated in rodent mammary tumor models grown in the mammary fat pads (Betof et al., 2015; Lee et al., 2015).
Figure 9.
Blood flow measured at rest and during exercise in rat prostate or rat prostate tumor tissue. *p<0.05 tumor in exercised vs resting host; †p<0.05 tumor vs prostate in exercised host. A two-way analysis of variance with repeated measures was used to compare within-group (rest vs exercise) and between-group (control vs tumor-bearing) differences in blood flow. Modified from McCullough et al. (2014). Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. Journal of the National Cancer Institute, 106, dju036.
Figure 10.
Representative fluorescence photomicrograph of a prostate tumor cross section following injection of EF5 (red; 30 mg/kg) and Hoechst-33342 (blue; 40 mg/kg) to illustrate hypoxia (red) and patent vessels (blue) in sedentary (A) and exercise-trained (B) orthotopic rat prostate tumors. C. and D. are graphic representations of the fraction of tissues bound by EF5 and patent vessel counts respectively. *p<0.05 between groups. Reprinted with permission from McCullough et al. (2013). Effects of exercise training on tumor hypoxia and vascular function in the rodent preclinical orthotopic prostate cancer model. Journal of Applied Physiology, 115, 1846–1854.
The diminished ability of the tumor vasculature to vasoconstrict in response to the systemic effects of exercise on arterial pressure, venous return and cardiac output, results in an enhanced delivery of oxygen to the tumor. Furthermore, tumor blood vessels in animals undergoing several weeks of exercise display increased stiffness (McCullough et al 2013) suggesting that such an exercise regimen may lead to normalization of the tumor vasculature and the establishment of more aerobic microenvironments. Although clearly encouraging, variables that may confound preclinical exercise and cancer results including intensity, duration, and type of exercise, as well as tumor models and animals used, remain to be thoroughly examined. Still, taken together, the application of aerobic exercise may provide a novel, non-toxic means to overcome tumor microenvironment-associated therapy resistance and hence improve cancer treatment outcomes.
5.0. Summary and perspectives
Solid tumor masses typically exhibit abnormal blood vessel networks associated with heterogeneous blood flow. A consequence of the spatial and temporal heterogeneity of the microcirculation is a failure to provide adequate and homogeneous nutritional support to all regions of the tumor. Cancer cells existing in areas of poor oxygenation, acidic pH, and high interstitial pressure not only display resistance to radiation and chemotherapy but emerging evidence suggests that such microenvironmental conditions may also profoundly affect the efficacy of cell signaling target interventions and immunotherapy.
Not only does the microenvironment participate in therapeutic resistance, but molecular investigations have revealed other important consequences in tumors. For example, stress responses to hypoxia involve mechanisms that clearly favor cell survival and malignant progression. Furthermore, molecular links between hypoxia and invasive, metastatic cancer cell characteristics continue to emerge. Given that upwards of 90% of cancer patient deaths are due to metastasis (McCord et al., 2009; Sleeman & Steeg, 2010; Siegel et al., 2012) coupled with the growing body of evidence that hypoxia exacerbates the metastatic phenotype, the ramifications for cancer patient treatment outcomes are severe. Consequently, therapeutic intervention strategies designed to overcome deleterious tumor microenvironments are clearly needed to provide more effective treatment options for cancer patients. Given the significant heterogeneity in tumor perfusion and oxygenation observed in human tumors it is paramount that such initiatives incorporate methodologies such as non-invasive imaging, immunohistochemical staining, and/or molecular signatures to evaluate individual tumors and identify patients at risk prior to initiation of the treatment. We believe that the development of novel treatment strategies aimed at turning the pathophysiological characteristics that distinguish tumors from normal tissues and confer treatment resistance into a therapeutic advantage will provide unique opportunities to overcome cancer treatment failures and improve patient care.
Acknowledgments
The authors’ work is supported by the National Cancer Institute (Public Health Service grant R01 CA169300) as well as the Danish Cancer Society and the Danish Council for Independent Research: Medical Sciences.
Abbreviations
- IFP
interstitial fluid pressure
- HIF-1
hypoxia inducible factor 1
- VEGF
vascular endothelial growth factor
- TIC
tumor initiating cell
- CTSL
cysteine protease cathepsin L
- CTL
cytotoxic T lymphocyte
- PD-L1
programmed cell death ligand-1
- AI
angiogenesis inhibitor
- VDA
vascular disrupting agent
- EPO
erythropoietin
- HCR
hypoxic cytotoxic ratio
Footnotes
Conflict of Interest
The authors declare that there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adams GE, Cooke MS. Electron-affinic sensitization. I. A structural basis for chemical radiosensitizers in bacteria. International Journal of Radiation Biology and Related Studies in Physics, Chemistry, and Medicine. 1969;15:457–471. doi: 10.1080/09553006914550741. [DOI] [PubMed] [Google Scholar]
- Adams GE, Flockhart IR, Smithen CE, Stratford IJ, Wardman P, Watts ME. Electron-affinic sensitization. VII. A correlation between structures, one-electron reduction potentials, and efficiencies of nitroimidazoles as hypoxic cell radiosensitizers. Radiation Research. 1976;67:9–20. [PubMed] [Google Scholar]
- Ahn GO, Brown M. Targeting tumors with hypoxia-activated cytotoxins. Frontiers in Bioscience. 2007;12:3483–3501. doi: 10.2741/2329. [DOI] [PubMed] [Google Scholar]
- Alqawi O, Wang HP, Espiritu M, Singh G. Chronic hypoxia promotes an aggressive phenotype in rat prostate cancer cells. Free Radical Research. 2007;41:788–797. doi: 10.1080/10715760701361531. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Laughlin MH. Rat muscle blood flows during high-speed locomotion. Journal of Applied Physiology. 1985;59:1322–1328. doi: 10.1152/jappl.1985.59.4.1322. [DOI] [PubMed] [Google Scholar]
- Bae KM, Su Z, Frye C, McClellan S, Allan RW, Andrejewski JT, Kelley V, Jorgensen M, Steindler DA, Vieweg J, Siemann DW. Expression of pluripotent stem cell reprogramming factors by prostate tumor initiating cells. The Journal of Urology. 2010;183:2045–2053. doi: 10.1016/j.juro.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F. Cancer and the chemokine network. Nature Reviews Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Research. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- Batchelor TT, Gerstner ER, Emblem KE, Duda DG, Kalpathy-Cramer J, Snuderl M, Ancukiewicz M, Polaskova P, Pinho MC, Jennings D, Plotkin SR, Chi AS, Eichler AF, Dietrich J, Hochberg FH, Lu-Emerson C, Iafrate AJ, Ivy SP, Rosen BR, Loeffler JS, Wen PY, Sorensen AG, Jain RK. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19059–19064. doi: 10.1073/pnas.1318022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nature Reviews Cancer. 2008;8:545–554. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- Bayer C, Shi K, Astner ST, Maftei CA, Vaupel P. Acute versus chronic hypoxia: why a simplified classification is simply not enough. International Journal of Radiation Oncology, Biology, Physics. 2011;80:965–968. doi: 10.1016/j.ijrobp.2011.02.049. [DOI] [PubMed] [Google Scholar]
- Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nature Genetics. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nature Reviews Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Bergsjo P, Kolstad P. Clinical trial with atmospheric oxygen breathing during radiotherapy of cancer of the cervix. Scandinavian Journal of Clinical and Laboratory Investigation Supplementum. 1968;106:167–171. [PubMed] [Google Scholar]
- Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR, Palmer G, Jones LW, Dewhirst MW. Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. Journal of the National Cancer Institute. 2015;107:djv040. doi: 10.1093/jnci/djv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy JL, Fox SB, Han C, Campo L, Turley H, Kanga S, Malone PR, Harris AL. The androgen receptor is significantly associated with vascular endothelial growth factor and hypoxia sensing via hypoxia-inducible factors HIF-1a, HIF-2a, and the prolyl hydroxylases in human prostate cancer. Clinical Cancer Research. 2005;11:7658–7663. doi: 10.1158/1078-0432.CCR-05-0460. [DOI] [PubMed] [Google Scholar]
- Boucher Y, Baxter LT, Jain RK. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Research. 1990;50:4478–4484. [PubMed] [Google Scholar]
- Breidahl T, Nielsen FU, Stodkilde-Jorgensen H, Maxwell RJ, Horsman MR. The effects of the vascular disrupting agents combretastatin A-4 disodium phosphate, 5,6-dimethylxanthenone-4-acetic acid and ZD6126 in a murine tumour: a comparative assessment using MRI and MRS. Acta Oncologica. 2006;45:306–316. doi: 10.1080/02841860600570465. [DOI] [PubMed] [Google Scholar]
- Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nature Reviews Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, Mueller-Klieser W. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. International Journal of Radiation Oncology, Biology, Physics. 2001;51:349–353. doi: 10.1016/s0360-3016(01)01630-3. [DOI] [PubMed] [Google Scholar]
- Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. International Journal of Radiation Oncology, Biology, Physics. 1997;38:285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- Brown JC, Winters-Stone K, Lee A, Schmitz KH. Cancer, physical activity, and exercise. Comprehensive Physiology. 2012;2:2775–2809. doi: 10.1002/cphy.c120005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM. The potential benefit of hypoxic cytotoxins in radio-oncology. In: Molls M, Vaupel P, editors. Medical Radiology. Heidelberg: Springer-Verlag; 1998. pp. 219–229. [Google Scholar]
- Burger JA, Stewart DJ, Wald O, Peled A. Potential of CXCR4 antagonists for the treatment of metastatic lung cancer. Expert Review of Anticancer Therapy. 2011;11:621–630. doi: 10.1586/era.11.11. [DOI] [PubMed] [Google Scholar]
- Cairns RA, Hill RP. Acute hypoxia enhances spontaneous lymph node metastasis in an orthotopic murine model of human cervical carcinoma. Cancer Research. 2004;64:2054–2061. doi: 10.1158/0008-5472.can-03-3196. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Adult participation in aerobic and muscle-strengthening physical activities--United States, 2011. Morbidity and Mortality Weekly Report. 2013;62:326–330. [PMC free article] [PubMed] [Google Scholar]
- Chan N, Bristow RG. “Contextual” synthetic lethality and/or loss of heterozygosity: tumor hypoxia and modification of DNA repair. Clinical Cancer Research. 2010;16:4553–4560. doi: 10.1158/1078-0432.CCR-10-0527. [DOI] [PubMed] [Google Scholar]
- Chaplin DJ, Horsman MR, Siemann DW. Further evaluation of nicotinamide and carbogen as a strategy to reoxygenate hypoxic cells in vivo: importance of nicotinamide dose and pre-irradiation breathing time. British journal of cancer. 1993;68:269–273. doi: 10.1038/bjc.1993.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin DJ, Olive PL, Durand RE. Intermittent blood flow in a murine tumor: radiobiological effects. Cancer Research. 1987;47:597–601. [PubMed] [Google Scholar]
- Chaudary N, Hill RP. Hypoxia and metastasis in breast cancer. Breast Disease. 2006;26:55–64. doi: 10.3233/bd-2007-26105. [DOI] [PubMed] [Google Scholar]
- Chaudary N, Hill RP. Hypoxia and metastasis. Clinical Cancer Research. 2007;13:1947–1949. doi: 10.1158/1078-0432.CCR-06-2971. [DOI] [PubMed] [Google Scholar]
- Chauhan SS, Goldstein LJ, Gottesman MM. Expression of cathepsin L in human tumors. Cancer Research. 1991;51:1478–1481. [PubMed] [Google Scholar]
- Cheng FC, Sheu ML, Su HL, Chen YJ, Chen CJ, Chiu WT, Sheehan J, Pan HC. The effect of exercise on mobilization of hematopoietic progenitor cells involved in the repair of sciatic nerve crush injury. Journal of neurosurgery. 2013;118:594–605. doi: 10.3171/2012.8.JNS111580. [DOI] [PubMed] [Google Scholar]
- Cherry T. A theory of cancer. Medical Journal of Australia. 1922;1:425–438. [Google Scholar]
- Churchill-Davidson I. The oxygen effect in radiotherapy: historical review. Frontiers of Radiation Therapeutic Oncology. 1968;1:1–15. [Google Scholar]
- Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes & Development. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen RL, Patterson AV, Telfer BA, Airley RE, Hobbs S, Phillips RM, Jaffar M, Stratford IJ, Williams KJ. Viral delivery of P450 reductase recapitulates the ability of constitutive overexpression of reductase enzymes to potentiate the activity of mitomycin C in human breast cancer xenografts. Molecular Cancer Therapeutics. 2003;2:901–909. [PubMed] [Google Scholar]
- Dai Y, Bae K, Pampo C, Siemann DW. Impact of the small molecule Met inhibitor BMS-777607 on the metastatic process in a rodent tumor model with constitutive c-Met activation. Clinical & Experimental Metastasis. 2012;29:253–261. doi: 10.1007/s10585-011-9447-z. [DOI] [PubMed] [Google Scholar]
- Dai Y, Bae K, Siemann DW. Impact of hypoxia on the metastatic potential of human prostate cancer cells. International Journal of Radiation Oncology, Biology, Physics. 2011;81:521–528. doi: 10.1016/j.ijrobp.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denekamp J, Hobson B. Endothelial-cell proliferation in experimental tumours. British Journal of Cancer. 1982;46:711–720. doi: 10.1038/bjc.1982.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny WA. The role of hypoxia-activated prodrugs in cancer therapy. The Lancet Oncology. 2000;1:25–29. doi: 10.1016/S1470-2045(00)00006-1. [DOI] [PubMed] [Google Scholar]
- Digklia A, Voutsadakis IA. Combinations of vascular endothelial growth factor pathway inhibitors with metronomic chemotherapy: Rational and current status. World journal of experimental medicine. 2014;4:58–67. doi: 10.5493/wjem.v4.i4.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dische S. Hyperbaric oxygen: the Medical Research Council trials and their clinical significance. British Journal of Radiology. 1978;51:888–894. doi: 10.1259/0007-1285-51-611-888. [DOI] [PubMed] [Google Scholar]
- Dische S, Chassagne D, Hope-Stone HF, Dawes PJDK, Roberts JT, Yosef H, Bey P, Horiot JC, Jacobson A, Frankendal B, Gonzales DG, Nguyen TD, Daly NJ, Le Floch O, Newman H, Vieiro E, Bennett MH, Bichel P, Duvillard P, Jacobson A. A trial of Ro 03-8799 (pimonidazole) in carcinoma of the uterine cervix: an interim report from the Medical Research Council Working Party on advanced carcinoma of the cervix. Radiotherapy and Oncology. 1993;26:93–103. [PubMed] [Google Scholar]
- Dobrowsky W, Huigol NG, Jayatilake RS, Kizilbash NI, Okkan S, Kagiya VT, Tatsuzaki H. AK-2123 (Sanazol) as a radiation sensitizer in the treatment of stage III cervical cancer: results of an IAEA multicentre randomised trial. Radiotherapy and Oncology. 2007;82:24–29. doi: 10.1016/j.radonc.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Drevs J, Muller-Driver R, Wittig C, Fuxius S, Esser N, Hugenschmidt H, Konerding MA, Allegrini PR, Wood J, Hennig J, Unger C, Marme D. PTK787/ZK 222584, a specific vascular endothelial growth factor-receptor tyrosine kinase inhibitor, affects the anatomy of the tumor vascular bed and the functional vascular properties as detected by dynamic enhanced magnetic resonance imaging. Cancer Research. 2002;62:4015–4022. [PubMed] [Google Scholar]
- Duan JX, Jiao H, Kaizerman J, Stanton T, Evans JW, Lan L, Lorente G, Banica M, Jung D, Wang J, Ma H, Li X, Yang Z, Hoffman RM, Ammons WS, Hart CP, Matteucci M. Potent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugs. Journal of Medicinal Chemistry. 2008;51:2412–2420. doi: 10.1021/jm701028q. [DOI] [PubMed] [Google Scholar]
- Dusault LA. The Effect of Oxygen on the Response of Spontaneous Tumours in Mice to Radiotherapy. The British Journal of Radiology. 1963;36:749–754. doi: 10.1259/0007-1285-36-430-749. [DOI] [PubMed] [Google Scholar]
- Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikesdal HP, Landuyt W, Dahl O. The influence of combretastatin A-4 and vinblastine on interstitial fluid pressure in BT4An rat gliomas. Cancer Letters. 2002;178:209–217. doi: 10.1016/s0304-3835(01)00835-7. [DOI] [PubMed] [Google Scholar]
- Eschwege F, Sancho-Garnier H, Chassagne D, Brisgand D, Guerra M, Malaise EP, Bey P, Busutti L, Cionini L, N’Guyen T, Romanini A, Chavaudra J, Hill C. Results of a European randomized trial of Etanidazole combined with radiotherapy in head and neck carcinomas. International Journal of Radiation Oncology, Biology, Physics. 1997;39:275–281. doi: 10.1016/s0360-3016(97)00327-1. [DOI] [PubMed] [Google Scholar]
- Evans JC, Bergsjo P. The Influence of Anemia on the Results of Radiotherapy in Carcinoma of the Cervix. Radiology. 1965;84:709–717. doi: 10.1148/84.4.709. [DOI] [PubMed] [Google Scholar]
- Everts V, Korper W, Hoeben KA, Jansen ID, Bromme D, Cleutjens KB, Heeneman S, Peters C, Reinheckel T, Saftig P, Beertsen W. Osteoclastic bone degradation and the role of different cysteine proteinases and matrix metalloproteinases: differences between calvaria and long bone. Journal of Bone and Mineral Research. 2006;21:1399–1408. doi: 10.1359/jbmr.060614. [DOI] [PubMed] [Google Scholar]
- Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, Gully C, Gassner R, Lepperdinger G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- Fenton BM, Paoni SF, Ding I. Pathophysiological effects of vascular endothelial growth factor receptor-2-blocking antibody plus fractionated radiotherapy on murine mammary tumors. Cancer Research. 2004;64:5712–5719. doi: 10.1158/0008-5472.CAN-04-0434. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes memorial Award lecture. Cancer Research. 1986;46:467–473. [PubMed] [Google Scholar]
- Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. Journal of Cellular Biochemistry. 2007;101:937–949. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- Fyles A, Milosevic M, Hedley D, Pintilie M, Levin W, Manchul L, Hill RP. Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. Journal of Clinical Oncology. 2002;20:680–687. doi: 10.1200/JCO.2002.20.3.680. [DOI] [PubMed] [Google Scholar]
- Garfinkel L, Stellman SD. Mortality by relative weight and exercise. Cancer. 1988;62:1844–1850. doi: 10.1002/1097-0142(19881015)62:1+<1844::aid-cncr2820621328>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Generali D, Berruti A, Brizzi MP, Campo L, Bonardi S, Wigfield S, Bersiga A, Allevi G, Milani M, Aguggini S, Gandolfi V, Dogliotti L, Bottini A, Harris AL, Fox SB. Hypoxia-inducible factor-1alpha expression predicts a poor response to primary chemoendocrine therapy and disease-free survival in primary human breast cancer. Clinical Cancer Research. 2006;12:4562–4568. doi: 10.1158/1078-0432.CCR-05-2690. [DOI] [PubMed] [Google Scholar]
- Gerweck LE, Nygaard TG, Burlett M. Response of cells to hyperthermia under acute and chronic hypoxic conditions. Cancer Research. 1979;39:966–972. [PubMed] [Google Scholar]
- Gholamnezhad Z, Boskabady MH, Hosseini M, Sankian M, Khajavi Rad A. Evaluation of immune response after moderate and overtraining exercise in wistar rat. Iranian Journal of Basic Medical Sciences. 2014;17:1–8. [PMC free article] [PubMed] [Google Scholar]
- Giaccia AJ. Hypoxic Stress Proteins: Survival of the Fittest. Seminars in Radiation Oncology. 1996;6:46–58. doi: 10.1053/SRAO0060046. [DOI] [PubMed] [Google Scholar]
- Gocheva V, Zeng W, Ke D, Klimstra D, Reinheckel T, Peters C, Hanahan D, Joyce JA. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes & Development. 2006;20:543–556. doi: 10.1101/gad.1407406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Pottgen C, Stuben G, Havers W, Stuschke M, Schweigerer L. Arginine deiminase and other antiangiogenic agents inhibit unfavorable neuroblastoma growth: potentiation by irradiation. International Journal of Cancer. 2003;106:723–728. doi: 10.1002/ijc.11298. [DOI] [PubMed] [Google Scholar]
- Goretzki L, Schmitt M, Mann K, Calvete J, Chucholowski N, Kramer M, Gunzler WA, Janicke F, Graeff H. Effective activation of the proenzyme form of the urokinase-type plasminogen activator (pro-uPA) by the cysteine protease cathepsin L. FEBS Letters. 1992;297:112–118. doi: 10.1016/0014-5793(92)80339-i. [DOI] [PubMed] [Google Scholar]
- Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- Grau C, Horsman MR, Overgaard J. Improving the radiation response in a C3H mouse mammary carcinoma by normobaric oxygen or carbogen breathing. International Journal of Radiation Oncology, Biology, Physics. 1992;22:415–419. doi: 10.1016/0360-3016(92)90844-8. [DOI] [PubMed] [Google Scholar]
- Grau C, Overgaard J. Significance of haemoglobin cincentration for treatment outcome. In: Molls M, Vaupel P, editors. Medical Radiology: Blood Perfusion and Microenvironment of Human Tumours. Heidelberg: Springer-Verlag; 1998. pp. 101–112. [Google Scholar]
- Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. British Journal of Radiology. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, Watowich SS, Gallick GE. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005;24:3110–3120. doi: 10.1038/sj.onc.1208513. [DOI] [PubMed] [Google Scholar]
- Grogan M, Thomas GM, Melamed I, Wong FL, Pearcey RG, Joseph PK, Portelance L, Crook J, Jones KD. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer. 1999;86:1528–1536. doi: 10.1002/(sici)1097-0142(19991015)86:8<1528::aid-cncr20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Guise CP, Abbattista MR, Tipparaju SR, Lambie NK, Su J, Li D, Wilson WR, Dachs GU, Patterson AV. Diflavin oxidoreductases activate the bioreductive prodrug PR-104A under hypoxia. Molecular Pharmacology. 2012;81:31–40. doi: 10.1124/mol.111.073759. [DOI] [PubMed] [Google Scholar]
- Guise CP, Mowday AM, Ashoorzadeh A, Yuan R, Lin WH, Wu DH, Smaill JB, Patterson AV, Ding K. Bioreductive prodrugs as cancer therapeutics: targeting tumor hypoxia. Chinese Journal of Cancer. 2014;33:80–86. doi: 10.5732/cjc.012.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nature Medicine. 2009;15:1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- Gutmann R, Leunig M, Feyh J, Goetz AE, Messmer K, Kastenbauer E, Jain RK. Interstitial hypertension in head and neck tumors in patients: correlation with tumor size. Cancer Research. 1992;52:1993–1995. [PubMed] [Google Scholar]
- Hara S, Nakashiro K, Klosek SK, Ishikawa T, Shintani S, Hamakawa H. Hypoxia enhances c-Met/HGF receptor expression and signaling by activating HIF-1alpha in human salivary gland cancer cells. Oral Oncology. 2006;42:593–598. doi: 10.1016/j.oraloncology.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Hatfield SM, Kjaergaard J, Lukashev D, Schreiber TH, Belikoff B, Abbott R, Sethumadhavan S, Philbrook P, Ko K, Cannici R, Thayer M, Rodig S, Kutok JL, Jackson EK, Karger B, Podack ER, Ohta A, Sitkovsky MV. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Science Translational Medicine. 2015;7:277ra230. doi: 10.1126/scitranslmed.aaa1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nature Medicine. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- Hiraoka M, Hahn GM. Changes in pH and blood flow induced by glucose, and their effects on hyperthermia with or without BCNU in RIF-1 tumours. International Journal of Hyperthermia. 1990;6:97–103. doi: 10.3109/02656739009140807. [DOI] [PubMed] [Google Scholar]
- Hirst DG, Wood PJ. Could manipulation of the binding affinity of haemoglobin for oxygen be used clinically to sensitize tumours to radiation? Radiotherapy and Oncology. 1991;20(Suppl 1):53–57. doi: 10.1016/0167-8140(91)90188-m. [DOI] [PubMed] [Google Scholar]
- Hockel M, Knoop C, Schlenger K, Vorndran B, Baussmann E, Mitze M, Knapstein PG, Vaupel P. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiotherapy and Oncology. 1993;26:45–50. doi: 10.1016/0167-8140(93)90025-4. [DOI] [PubMed] [Google Scholar]
- Hoff CM. Importance of hemoglobin concentration and its modification for the outcome of head and neck cancer patients treated with radiotherapy. Acta Oncologica. 2012;51:419–432. doi: 10.3109/0284186X.2011.653438. [DOI] [PubMed] [Google Scholar]
- Horsman MR. Nicotinamide and other benzamide analogs as agents for overcoming hypoxic cell radiation resistance in tumours. A review. Acta Oncologica. 1995;34:571–587. doi: 10.3109/02841869509094031. [DOI] [PubMed] [Google Scholar]
- Horsman MR. Measurement of tumor oxygenation. International Journal of Radiation Oncology, Biology, Physics. 1998;42:701–704. doi: 10.1016/s0360-3016(98)00332-0. [DOI] [PubMed] [Google Scholar]
- Horsman MR. Tissue physiology and the response to heat. International Journal of Hyperthermia. 2006;22:197–203. doi: 10.1080/02656730600689066. [DOI] [PubMed] [Google Scholar]
- Horsman MR, Christensen KL, Overgaard J. Hydralazine-induced enhancement of hyperthermic damage in a C3H mammary carcinoma in vivo. International Journal of Hyperthermia. 1989;5:123–136. doi: 10.3109/02656738909140442. [DOI] [PubMed] [Google Scholar]
- Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nature Reviews Clinical Oncology. 2012;9:674–687. doi: 10.1038/nrclinonc.2012.171. [DOI] [PubMed] [Google Scholar]
- Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clinical Oncology. 2007;19:418–426. doi: 10.1016/j.clon.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Horsman MR, Overgaard J, Siemann DW. Impact on radiotherapy. In: Siemann DW, editor. Tumor Microenvironment. Chichester: John Wiley & Sons, Ltd; 2011. pp. 353–376. [Google Scholar]
- Horsman MR, Siemann DW. Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Research. 2006;66:11520–11539. doi: 10.1158/0008-5472.CAN-06-2848. [DOI] [PubMed] [Google Scholar]
- Hoskin PJ, Robinson M, Slevin N, Morgan D, Harrington K, Gaffney C. Effect of epoetin alfa on survival and cancer treatment-related anemia and fatigue in patients receiving radical radiotherapy with curative intent for head and neck cancer. Journal of Clinical Oncology. 2009;27:5751–5756. doi: 10.1200/JCO.2009.22.3693. [DOI] [PubMed] [Google Scholar]
- Hoskin PJ, Rojas AM, Bentzen SM, Saunders MI. Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. Journal of Clinical Oncology. 2010;28:4912–4918. doi: 10.1200/JCO.2010.28.4950. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Chang HJ, Shim YH, Park WH, Park W, Huh SJ, Yang JH. Effects of supervised exercise therapy in patients receiving radiotherapy for breast cancer. Yonsei Medical Journal. 2008;49:443–450. doi: 10.3349/ymj.2008.49.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen AB, Busk M, Horsman MR. Induction of hypoxia by vascular disrupting agents and the significance for their combination with radiation therapy. Acta Oncologica. 2013;52:1320–1326. doi: 10.3109/0284186X.2013.825050. [DOI] [PubMed] [Google Scholar]
- Jagodic M, Vrhovec I, Borstnar S, Cufer T. Prognostic and predictive value of cathepsins D and L in operable breast cancer patients. Neoplasma. 2005;52:1–9. [PubMed] [Google Scholar]
- Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nature Medicine. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens GO, Rademakers SE, Terhaard CH, Doornaert PA, Bijl HP, van den Ende P, Chin A, Marres HA, de Bree R, van der Kogel AJ, Hoogsteen IJ, Bussink J, Span PN, Kaanders JH. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: results of a phase III randomized trial. Journal of Clinical Oncology. 2012;30:1777–1783. doi: 10.1200/JCO.2011.35.9315. [DOI] [PubMed] [Google Scholar]
- Jean D, Rousselet N, Frade R. Cathepsin L expression is up-regulated by hypoxia in human melanoma cells: role of its 5′-untranslated region. The Biochemical Journal. 2008;413:125–134. doi: 10.1042/BJ20071255. [DOI] [PubMed] [Google Scholar]
- Jones LW, Antonelli J, Masko EM, Broadwater G, Lascola CD, Fels D, Dewhirst MW, Dyck JR, Nagendran J, Flores CT, Betof AS, Nelson ER, Pollak M, Dash RC, Young ME, Freedland SJ. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. Journal of Applied Physiology. 2012;113:263–272. doi: 10.1152/japplphysiol.01575.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CT, Guzman ML, Noble M. Cancer stem cells. The New England Journal of Medicine. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- Kaanders JH, Bussink J, van der Kogel AJ. ARCON: a novel biology-based approach in radiotherapy. The Lancet Oncology. 2002;3:728–737. doi: 10.1016/s1470-2045(02)00929-4. [DOI] [PubMed] [Google Scholar]
- Kaelin WG., Jr The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nature Reviews Cancer. 2008;8:865–873. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. International Journal of Radiation Biology. 2001;77:399–408. doi: 10.1080/09553000010024687. [DOI] [PubMed] [Google Scholar]
- Karasawa K, Sunamura M, Okamoto A, Nemoto K, Matsuno S, Nishimura Y, Shibamoto Y. Efficacy of novel hypoxic cell sensitiser doranidazole in the treatment of locally advanced pancreatic cancer: long-term results of a placebo-controlled randomised study. Radiotherapy and Oncology. 2008;87:326–330. doi: 10.1016/j.radonc.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nature Reviews Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nature reviews Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- Kimbro KS, Simons JW. Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocrine-related Cancer. 2006;13:739–749. doi: 10.1677/erc.1.00728. [DOI] [PubMed] [Google Scholar]
- Kimura H, Braun RD, Ong ET, Hsu R, Secomb TW, Papahadjopoulos D, Hong K, Dewhirst MW. Fluctuations in red cell flux in tumor microvessels can lead to transient hypoxia and reoxygenation in tumor parenchyma. Cancer Research. 1996;56:5522–5528. [PubMed] [Google Scholar]
- Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Cosmatos D, Marcial VA, Fu KK, Rotman M, Cooper JS, Ortiz HG, Beitler JJ, Abrams RA, Curran WJ, et al. Results of an RTOG phase III trial (RTOG 85-27) comparing radiotherapy plus etanidazole with radiotherapy alone for locally advanced head and neck carcinomas. International Journal of Radiation Oncology, Biology, Physics. 1995;32:567–576. doi: 10.1016/0360-3016(95)00150-W. [DOI] [PubMed] [Google Scholar]
- Lee JA, Biel NM, Kozikowski RT, Siemann DW, Sorg BS. In vivo spectral and fluorescence microscopy comparison of microvascular function after treatment with OXi4503, Sunitinib and their combination in Caki-2 tumors. Biomedical Optics Express. 2014;5:1965–1979. doi: 10.1364/BOE.5.001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Wiggins J, Siemann DW. Impact of aerobic exercise on tumor oxygenation and perfusion in breast cancer [abstract]. Proceedings of the American Association for Cancer Research Special Conference on Tumor Angiogenesis and Vascular Normalization: Bench to Bedside to Biomarkers; Orlando, FL. Philadelphia (PA): AACR; 2015. (Abstract nr B24) [Google Scholar]
- Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, Hjelmeland AB, Rich JN. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis JM, Buricchi F, Chiarugi P, Morales A, Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-{kappa}B via c-SRC and oxidant-dependent cell death. Cancer Research. 2007;67:7368–7377. doi: 10.1158/0008-5472.CAN-07-0515. [DOI] [PubMed] [Google Scholar]
- Lunt SJ, Telfer BA, Fitzmaurice RJ, Stratford IJ, Williams KJ. Tirapazamine administered as a neoadjuvant to radiotherapy reduces metastatic dissemination. Clinical Cancer Research. 2005;11:4212–4216. doi: 10.1158/1078-0432.CCR-04-2162. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008;371:771–783. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]
- Mathieu J, Zhang Z, Zhou W, Wang AJ, Heddleston JM, Pinna CM, Hubaud A, Stadler B, Choi M, Bar M, Tewari M, Liu A, Vessella R, Rostomily R, Born D, Horwitz M, Ware C, Blau CA, Cleary MA, Rich JN, Ruohola-Baker H. HIF induces human embryonic stem cell markers in cancer cells. Cancer Research. 2011;71:4640–4652. doi: 10.1158/0008-5472.CAN-10-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr NA, Wang JZ, Zhang D, Grecula JC, Lo SS, Jaroura D, Montebello J, Zhang H, Li K, Lu L, Huang Z, Fowler JM, Wu DH, Knopp MV, Yuh WT. Longitudinal changes in tumor perfusion pattern during the radiation therapy course and its clinical impact in cervical cancer. International Journal of Radiation Oncology, Biology, Physics. 2010;77:502–508. doi: 10.1016/j.ijrobp.2009.04.084. [DOI] [PubMed] [Google Scholar]
- McCord AM, Jamal M, Shankavaram UT, Lang FF, Camphausen K, Tofilon PJ. Physiologic oxygen concentration enhances the stem-like properties of CD133+ human glioblastoma cells in vitro. Molecular Cancer Research. 2009;7:489–497. doi: 10.1158/1541-7786.MCR-08-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough DJ, Nguyen LM, Siemann DW, Behnke BJ. Effects of exercise training on tumor hypoxia and vascular function in the rodent preclinical orthotopic prostate cancer model. Journal of Applied Physiology. 2013;115:1846–1854. doi: 10.1152/japplphysiol.00949.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough DJ, Stabley JN, Siemann DW, Behnke BJ. Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. Journal of the National Cancer Institute. 2014;106:dju036. doi: 10.1093/jnci/dju036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown SR, Cowen RL, Williams KJ. Bioreductive drugs: from concept to clinic. Clinical Oncology. 2007;19:427–442. doi: 10.1016/j.clon.2007.03.006. [DOI] [PubMed] [Google Scholar]
- McKeown SR, Hejmadi MV, McIntyre IA, McAleer JJ, Patterson LH. AQ4N: an alkylaminoanthraquinone N-oxide showing bioreductive potential and positive interaction with radiation in vivo. British Journal of Cancer. 1995;72:76–81. doi: 10.1038/bjc.1995.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall WM, Morris CG, Amdur RJ, Mendenhall NP, Siemann DW. Radiotherapy alone or combined with carbogen breathing for squamous cell carcinoma of the head and neck: a prospective, randomized trial. Cancer. 2005;104:332–337. doi: 10.1002/cncr.21146. [DOI] [PubMed] [Google Scholar]
- Meng F, Evans JW, Bhupathi D, Banica M, Lan L, Lorente G, Duan JX, Cai X, Mowday AM, Guise CP, Maroz A, Anderson RF, Patterson AV, Stachelek GC, Glazer PM, Matteucci MD, Hart CP. Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302. Molecular Cancer Therapeutics. 2012;11:740–751. doi: 10.1158/1535-7163.MCT-11-0634. [DOI] [PubMed] [Google Scholar]
- Milecki P, Hojan K, Ozga-Majchrzak O, Molinska-Glura M. Exercise tolerance in breast cancer patients during radiotherapy after aerobic training. Contemporary Oncology. 2013;17:205–209. doi: 10.5114/wo.2013.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosevic M, Chung P, Parker C, Bristow R, Toi A, Panzarella T, Warde P, Catton C, Menard C, Bayley A, Gospodarowicz M, Hill R. Androgen withdrawal in patients reduces prostate cancer hypoxia: implications for disease progression and radiation response. Cancer Research. 2007;67:6022–6025. doi: 10.1158/0008-5472.CAN-07-0561. [DOI] [PubMed] [Google Scholar]
- Milosevic M, Fyles A, Hedley D, Pintilie M, Levin W, Manchul L, Hill R. Interstitial fluid pressure predicts survival in patients with cervix cancer independent of clinical prognostic factors and tumor oxygen measurements. Cancer Research. 2001;61:6400–6405. [PubMed] [Google Scholar]
- Milosevic M, Warde P, Menard C, Chung P, Toi A, Ishkanian A, McLean M, Pintilie M, Sykes J, Gospodarowicz M, Catton C, Hill RP, Bristow R. Tumor hypoxia predicts biochemical failure following radiotherapy for clinically localized prostate cancer. Clinical Cancer Research. 2012;18:2108–2114. doi: 10.1158/1078-0432.CCR-11-2711. [DOI] [PubMed] [Google Scholar]
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nature reviews Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- Monk B, Sill M, Walker J, DiSilvestro P, Sutton G, Tewari K, Pajon E, Martin L, Schilder J, Coleman R, Balkssoon J, Aghajanian C. Randomized phase 2 evaluation of bevacizumab versus bevacizumab/fosbretabulin in recurrent ovarian, tubal or peritoneal carcinoma: a gynecologic oncology group study [abstract] International Journal of Gynecological Cancer. 2014;24(suppl 4):42–43. [Google Scholar]
- Mortensen LS, Johansen J, Kallehauge J, Primdahl H, Busk M, Lassen P, Alsner J, Sorensen BS, Toustrup K, Jakobsen S, Petersen J, Petersen H, Theil J, Nordsmark M, Overgaard J. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiotherapy and Oncology. 2012;105:14–20. doi: 10.1016/j.radonc.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Moulder JE, Rockwell S. Hypoxic fractions of solid tumors: experimental techniques, methods of analysis, and a survey of existing data. International Journal of Radiation Oncology, Biology, Physics. 1984;10:695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- Movsas B, Chapman JD, Hanlon AL, Horwitz EM, Greenberg RE, Stobbe C, Hanks GE, Pollack A. Hypoxic prostate/muscle pO2 ratio predicts for biochemical failure in patients with prostate cancer: preliminary findings. Urology. 2002;60:634–639. doi: 10.1016/s0090-4295(02)01858-7. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP. Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature. 1995;375:577–581. doi: 10.1038/375577a0. [DOI] [PubMed] [Google Scholar]
- Mustian KM, Peppone L, Darling TV, Palesh O, Heckler CE, Morrow GR. A 4-week home-based aerobic and resistance exercise program during radiation therapy: a pilot randomized clinical trial. Journal of Supportive Oncology. 2009;7:158–167. [PMC free article] [PubMed] [Google Scholar]
- Nielsen T, Murata R, Maxwell RJ, Stodkilde-Jorgensen H, Ostergaard L, Ley CD, Kristjansen PE, Horsman MR. Non-invasive imaging of combretastatin activity in two tumor models: Association with invasive estimates. Acta Oncologica. 2010;49:906–913. doi: 10.3109/0284186X.2010.499135. [DOI] [PubMed] [Google Scholar]
- Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, Becker A, Adam M, Molls M, Dunst J, Terris DJ, Overgaard J. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiotherapy and Oncology. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Overgaard J. Sensitization of hypoxic tumour cells--clinical experience. International Journal of Radiation Biology. 1989;56:801–811. doi: 10.1080/09553008914552081. [DOI] [PubMed] [Google Scholar]
- Overgaard J. Clinical evaluation of nitroimidazoles as modifiers of hypoxia in solid tumors. Oncology Research. 1994;6:509–518. [PubMed] [Google Scholar]
- Overgaard J, Bichel P. The influence of hypoxia and acidity on the hyperthermic response of malignant cells in vitro. Radiology. 1977;123:511–514. doi: 10.1148/123.2.511. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, Lindelov B, Jorgensen K. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5–85. Radiotherapy and Oncology. 1998;46:135–146. doi: 10.1016/s0167-8140(97)00220-x. [DOI] [PubMed] [Google Scholar]
- Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffenbarger RS, Jr, Hyde RT, Wing AL. Physical activity and incidence of cancer in diverse populations: a preliminary report. American Journal of Clinical Nutrition. 1987;45:312–317. doi: 10.1093/ajcn/45.1.312. [DOI] [PubMed] [Google Scholar]
- Patterson AV, Ferry DM, Edmunds SJ, Gu Y, Singleton RS, Patel K, Pullen SM, Hicks KO, Syddall SP, Atwell GJ, Yang S, Denny WA, Wilson WR. Mechanism of action and preclinical antitumor activity of the novel hypoxia-activated DNA cross-linking agent PR-104. Clinical Cancer Research. 2007;13:3922–3932. doi: 10.1158/1078-0432.CCR-07-0478. [DOI] [PubMed] [Google Scholar]
- Patterson LH, McKeown SR. AQ4N: a new approach to hypoxia-activated cancer chemotherapy. British Journal of Cancer. 2000;83:1589–1593. doi: 10.1054/bjoc.2000.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiological Reviews. 2000;80:1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Petersen C, Zips D, Krause M, Schone K, Eicheler W, Hoinkis C, Thames HD, Baumann M. Repopulation of FaDu human squamous cell carcinoma during fractionated radiotherapy correlates with reoxygenation. International Journal of Radiation Oncology, Biology, Physics. 2001;51:483–493. doi: 10.1016/s0360-3016(01)01686-8. [DOI] [PubMed] [Google Scholar]
- Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, Kerbel RS. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. American Journal of Pathology. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends in Immunology. 2007;28:299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham NA, Magalhaes JM, Do T, Schwock J, Dhani N, Cao PJ, Hill RP, Hedley DW. Activation of Src and Src-associated signaling pathways in relation to hypoxia in human cancer xenograft models. International Journal of Cancer. 2009;124:280–286. doi: 10.1002/ijc.23912. [DOI] [PubMed] [Google Scholar]
- Phillips C. NCI Cancer Bulletin. Bethesda, MD: National Cancer Institute; 2010. Guidelines Urge Exercise for Cancer Patients, Survivors. [Google Scholar]
- Pitson G, Fyles A, Milosevic M, Wylie J, Pintilie M, Hill R. Tumor size and oxygenation are independent predictors of nodal diseases in patients with cervix cancer. International Journal of Radiation Oncology, Biology, Physics. 2001;51:699–703. doi: 10.1016/s0360-3016(01)01662-5. [DOI] [PubMed] [Google Scholar]
- Qing G, Simon MC. Hypoxia inducible factor-2alpha: a critical mediator of aggressive tumor phenotypes. Current Opinion in Genetics & Development. 2009;19:60–66. doi: 10.1016/j.gde.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers SE, Span PN, Kaanders JH, Sweep FC, van der Kogel AJ, Bussink J. Molecular aspects of tumour hypoxia. Molecular Oncology. 2008;2:41–53. doi: 10.1016/j.molonc.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauth AM, McClelland RA, Michaels HB, Battistella R. The oxygen dependence of the reduction of nitroimidazoles in a radiolytic model system. International Journal of Radiation Oncology, Biology, Physics. 1984;10:1323–1326. doi: 10.1016/0360-3016(84)90341-9. [DOI] [PubMed] [Google Scholar]
- Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, Da Violante G, Gourlaouen M, Salih M, Jones MC, Jones DT, Saunders G, Kostourou V, Perron-Sierra F, Norman JC, Tucker GC, Hodivala-Dilke KM. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nature Medicine. 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- Rice L, Lepler S, Pampo C, Siemann DW. Impact of the SRC inhibitor dasatinib on the metastatic phenotype of human prostate cancer cells. Clinical & Experimental Metastasis. 2012;29:133–142. doi: 10.1007/s10585-011-9436-2. [DOI] [PubMed] [Google Scholar]
- Rockwell S. Use of a perfluorochemical emulsion to improve oxygenation in a solid tumor. International Journal of Radiation Oncology, Biology, Physics. 1985;11:97–103. doi: 10.1016/0360-3016(85)90367-0. [DOI] [PubMed] [Google Scholar]
- Rockwell S. Effect of some proliferative and environmental factors on the toxicity of mitomycin C to tumor cells in vitro. International Journal of Cancer. 1986;38:229–235. doi: 10.1002/ijc.2910380213. [DOI] [PubMed] [Google Scholar]
- Rofstad EK, Galappathi K, Mathiesen B, Ruud EB. Fluctuating and diffusion-limited hypoxia in hypoxia-induced metastasis. Clinical Cancer Research. 2007;13:1971–1978. doi: 10.1158/1078-0432.CCR-06-1967. [DOI] [PubMed] [Google Scholar]
- Rojas A. Radiosensitization with normobaric oxygen and carbogen. Radiotherapy and Oncology. 1991;20(Suppl 1):65–70. doi: 10.1016/0167-8140(91)90190-r. [DOI] [PubMed] [Google Scholar]
- Romiti A, Cox MC, Sarcina I, Di Rocco R, D’Antonio C, Barucca V, Marchetti P. Metronomic chemotherapy for cancer treatment: a decade of clinical studies. Cancer Chemotherapy and Pharmacology. 2013;72:13–33. doi: 10.1007/s00280-013-2125-x. [DOI] [PubMed] [Google Scholar]
- Roti Roti JL. Introduction: radiosensitization by hyperthermia. International Journal of Hyperthermia. 2004;20:109–114. doi: 10.1080/0265673032000173898. [DOI] [PubMed] [Google Scholar]
- Rubin P, Hanley J, Keys HM, Marcial V, Brady L. Carbogen breathing during radiation therapy-the Radiation Therapy Oncology Group Study. International Journal of Radiation Oncology, Biology, Physics. 1979;5:1963–1970. doi: 10.1016/0360-3016(79)90946-5. [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y, Maehara Y, Baba H, Kusumoto T, Sugimachi K, Newman RA. Flavone acetic acid increases the antitumor effect of hyperthermia in mice. Cancer Research. 1992;52:3306–3309. [PubMed] [Google Scholar]
- Sartorelli AC. Therapeutic attack of hypoxic cells of solid tumors: presidential address. Cancer Research. 1988;48:775–778. [PubMed] [Google Scholar]
- Saunders MP, Jaffar M, Patterson AV, Nolan J, Naylor MA, Phillips RM, Harris AL, Stratford IJ. The relative importance of NADPH: cytochrome c (P450) reductase for determining the sensitivity of human tumour cells to the indolequinone EO9 and related analogues lacking functionality at the C-2 and C-3 positions. Biochemical Pharmacology. 2000;59:993–996. doi: 10.1016/s0006-2952(99)00405-0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Current Opinion in Genetics & Development. 2004;14:92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine and Science in Sports and Exercise. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nature Reviews Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a Cancer Journal for Clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Siemann DW. Chemosensitization of CCNU in KHT murine tumor cells in vivo and in vitro by the agent RB 6145 and its isomer PD 144872. Radiotherapy and Oncology. 1995;34:47–53. doi: 10.1016/0167-8140(94)01498-r. [DOI] [PubMed] [Google Scholar]
- Siemann DW. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treatment Reviews. 2011;37:63–74. doi: 10.1016/j.ctrv.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann DW, Alliet KL. Potentiation of CCNU activity by misonidazole in metastases. Clinical & Experimental Metastasis. 1987;5:57–63. doi: 10.1007/BF00116626. [DOI] [PubMed] [Google Scholar]
- Siemann DW, Dai Y, Horsman MR. Hypoxia, metastasis, and antiangiogenic therapies. In: Melillo G, editor. Hypoxia and Cancer: Biological Implications and Therapeutic Opportunities. New York: Springer; 2014. pp. 205–227. [Google Scholar]
- Siemann DW, Dong M, Pampo C, Shi W. Src-signaling interference impairs the dissemination of blood-borne tumor cells. Cell and Tissue Research. 2012;349:541–550. doi: 10.1007/s00441-012-1415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann DW, Hill RP, Bush RS. The importance of the pre-irradiation breathing times of oxygen and carbogen (5% CO2: 95% O2) on the in vivo radiation response of a murine sarcoma. International Journal of Radiation Oncology, Biology, Physics. 1977;2:903–911. doi: 10.1016/0360-3016(77)90188-2. [DOI] [PubMed] [Google Scholar]
- Siemann DW, Horsman MR. Vascular targeted therapies in oncology. Cell and Tissue Research. 2009;335:241–248. doi: 10.1007/s00441-008-0646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann DW, Macler LM. Tumor radiosensitization through reductions in hemoglobin affinity. International Journal of Radiation Oncology, Biology, Physics. 1986;12:1295–1297. doi: 10.1016/0360-3016(86)90157-4. [DOI] [PubMed] [Google Scholar]
- Siemann DW, Mulcahy RT. Sensitization of cancer chemotherapeutic agents by nitroheterocyclics. Biochemical Pharmacology. 1986;35:111–115. doi: 10.1016/0006-2952(86)90567-8. [DOI] [PubMed] [Google Scholar]
- Siemann DW, Shi W. Efficacy of combined antiangiogenic and vascular disrupting agents in treatment of solid tumors. International Journal of Radiation Oncology, Biology, Physics. 2004;60:1233–1240. doi: 10.1016/j.ijrobp.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Siemann DW, Shi W. Dual targeting of tumor vasculature: combining Avastin and vascular disrupting agents (CA4P or OXi4503) Anticancer Research. 2008;28:2027–2031. [PMC free article] [PubMed] [Google Scholar]
- Siemann DW, Warrington KH, Horsman MR. Targeting tumor blood vessels: an adjuvant strategy for radiation therapy. Radiotherapy and Oncology. 2000;57:5–12. doi: 10.1016/s0167-8140(00)00243-7. [DOI] [PubMed] [Google Scholar]
- Skliarenko JV, Lunt SJ, Gordon ML, Vitkin A, Milosevic M, Hill RP. Effects of the vascular disrupting agent ZD6126 on interstitial fluid pressure and cell survival in tumors. Cancer Research. 2006;66:2074–2080. doi: 10.1158/0008-5472.CAN-05-2046. [DOI] [PubMed] [Google Scholar]
- Sleeman J, Steeg PS. Cancer metastasis as a therapeutic target. European Journal of Cancer. 2010;46:1177–1180. doi: 10.1016/j.ejca.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nature Medicine. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- Stewart GD, Ross JA, McLaren DB, Parker CC, Habib FK, Riddick AC. The relevance of a hypoxic tumour microenvironment in prostate cancer. BJU International. 2010;105:8–13. doi: 10.1111/j.1464-410X.2009.08921.x. [DOI] [PubMed] [Google Scholar]
- Stratford IJ, O’Neill P, Sheldon PW, Silver AR, Walling JM, Adams GE. RSU 1069, a nitroimidazole containing an aziridine group. Bioreduction greatly increases cytotoxicity under hypoxic conditions. Biochemical Pharmacology. 1986;35:105–109. doi: 10.1016/0006-2952(86)90566-6. [DOI] [PubMed] [Google Scholar]
- Stratford IJ, Williams KJ, Cowen RL, Jaffar M. Combining bioreductive drugs and radiation for the treatment of solid tumors. Seminars in Radiation Oncology. 2003;13:42–52. doi: 10.1053/srao.2003.50008. [DOI] [PubMed] [Google Scholar]
- Sudhan DR, Rabaglino BM, Wood CE, Siemann DW. Role of Cathepsin L in breast cancer angiogenesis [abstract]. Proceedings of the American Association for Cancer Research Annual Meeting; Philadelphia (PA). 2015. (Abstract nr 4185) [Google Scholar]
- Sudhan DR, Siemann DW. Cathepsin L inhibition by the small molecule KGP94 suppresses tumor microenvironment enhanced metastasis associated cell functions of prostate and breast cancer cells. Clinical & Experimental Metastasis. 2013;30:891–902. doi: 10.1007/s10585-013-9590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suit HD, Marshall N, Woerner D. Oxygen, oxygen plus carbon dioxide, and radiation therapy of a mouse mammary carcinoma. Cancer. 1972;30:1154–1158. doi: 10.1002/1097-0142(197211)30:5<1154::aid-cncr2820300503>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Graham CH. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Reviews. 2007;26:319–331. doi: 10.1007/s10555-007-9062-2. [DOI] [PubMed] [Google Scholar]
- Sutherland RM, Keng P, Conroy PJ, McDermott D, Bareham BJ, Passalacqua W. In vitro hypoxic cytotoxicity of nitroimidazoles: uptake and cell cycle phase specificity. International Journal of Radiation Oncology, Biology, Physics. 1982;8:745–748. doi: 10.1016/0360-3016(82)90726-x. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Teicher BA, Holden SA, Ara G, Sotomayor EA, Huang ZD, Chen YN, Brem H. Potentiation of cytotoxic cancer therapies by TNP-470 alone and with other anti-angiogenic agents. International Journal of Cancer. 1994;57:920–925. doi: 10.1002/ijc.2910570624. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Thomas GM. Raising hemoglobin: an opportunity for increasing survival? Oncology. 2002;63(Suppl 2):19–28. doi: 10.1159/000067148. [DOI] [PubMed] [Google Scholar]
- Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. British Journal of Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Research. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- Torimura T, Iwamoto H, Nakamura T, Koga H, Ueno T, Kerbel RS, Sata M. Metronomic chemotherapy: possible clinical application in advanced hepatocellular carcinoma. Translational oncology. 2013;6:511–519. doi: 10.1593/tlo.13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nature Reviews Molecular Cell Biology. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- Turaka A, Buyyounouski MK, Hanlon AL, Horwitz EM, Greenberg RE, Movsas B. Hypoxic prostate/muscle PO2 ratio predicts for outcome in patients with localized prostate cancer: long-term results. International Journal of Radiation Oncology, Biology, Physics. 2012;82:e433–439. doi: 10.1016/j.ijrobp.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Urano M. Thermochemotherapy: from in vitro and in vivo experiments to potential clinical application. In: Urano M, Douple E, editors. Hyperthermia and Oncology. Utrecht, The Netherlands: VSP; 1994. pp. 169–204. [Google Scholar]
- Urtasun R, Band P, Chapman JD, Feldstein ML, Mielke B, Fryer C. Radiation and high-dose metronidazole in supratentorial glioblastomas. The New England Journal of Medicine. 1976;294:1364–1367. doi: 10.1056/NEJM197606172942503. [DOI] [PubMed] [Google Scholar]
- van Waart H, Stuiver MM, van Harten WH, Sonke GS, Aaronson NK. Design of the Physical exercise during Adjuvant Chemotherapy Effectiveness Study (PACES): a randomized controlled trial to evaluate effectiveness and cost-effectiveness of physical exercise in improving physical fitness and reducing fatigue. BMC Cancer. 2010;10:673. doi: 10.1186/1471-2407-10-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Seminars in Radiation Oncology. 2004;14:198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Vaupel P. Pathophysiology of solid tumors. In: Molls M, Vaupel P, Nieder C, Anscher MS, editors. The Impact of Tumor Biology on Cancer Treatment and Multidisciplinary Strategies. New York: Springer; 2009. pp. 51–92. [Google Scholar]
- Vaupel P. Pathophysiology of human tumors. In: Osinsky S, Friese H, Vaupel P, editors. Tumor Hypoxia in the Clinical Setting. Ukraine, Kiev: National Academy of Sciences; 2011. pp. 21–64. [Google Scholar]
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Research. 1989;49:6449–6465. [PubMed] [Google Scholar]
- Vaupel P, Osinsky S. Tumor hypoxia and acidosis, and the response to chemotherapy. In: Osinsky S, Friese H, Vaupel P, editors. Tumor Hypoxia in the Clinical Setting. Ukraine, Kiev: National Academy of Sciences; 2011. pp. 93–110. [Google Scholar]
- Voorhees WD, 3rd, Babbs CF. Hydralazine-enhanced selective heating of transmissible venereal tumor implants in dogs. European Journal of Cancer & Clinical Oncology. 1982;18:1027–1033. doi: 10.1016/0277-5379(82)90252-8. [DOI] [PubMed] [Google Scholar]
- Walenta S, Mueller-Klieser WF. Lactate: mirror and motor of tumor malignancy. Seminars in Radiation Oncology. 2004;14:267–274. doi: 10.1016/j.semradonc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nature Reviews Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- Windsor PM, Nicol KF, Potter J. A randomized, controlled trial of aerobic exercise for treatment-related fatigue in men receiving radical external beam radiotherapy for localized prostate carcinoma. Cancer. 2004;101:550–557. doi: 10.1002/cncr.20378. [DOI] [PubMed] [Google Scholar]
- Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333–344. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PP, Demircioglu F, Ghazaly E, Alrawashdeh W, Stratford MR, Scudamore CL, Cereser B, Crnogorac-Jurcevic T, McDonald S, Elia G, Hagemann T, Kocher HM, Hodivala-Dilke KM. Dual-action combination therapy enhances angiogenesis while reducing tumor growth and spread. Cancer Cell. 2015;27:123–137. doi: 10.1016/j.ccell.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Zajc I, Sever N, Bervar A, Lah TT. Expression of cysteine peptidase cathepsin L and its inhibitors stefins A and B in relation to tumorigenicity of breast cancer cell lines. Cancer Letters. 2002;187:185–190. doi: 10.1016/s0304-3835(02)00452-4. [DOI] [PubMed] [Google Scholar]
- Zannella VE, Dal Pra A, Muaddi H, McKee TD, Stapleton S, Sykes J, Glicksman R, Chaib S, Zamiara P, Milosevic M, Wouters BG, Bristow RG, Koritzinsky M. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clinical Cancer Research. 2013;19:6741–6750. doi: 10.1158/1078-0432.CCR-13-1787. [DOI] [PubMed] [Google Scholar]
- Zeman EM, Brown JM, Lemmon MJ, Hirst VK, Lee WW. SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells. International Journal of Radiation Oncology, Biology, Physics. 1986;12:1239–1242. doi: 10.1016/0360-3016(86)90267-1. [DOI] [PubMed] [Google Scholar]
- Zhang L, Huang G, Li X, Zhang Y, Jiang Y, Shen J, Liu J, Wang Q, Zhu J, Feng X, Dong J, Qian C. Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor -1alpha in hepatocellular carcinoma. BMC cancer. 2013;13:108. doi: 10.1186/1471-2407-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiology. 2013;37:207–218. doi: 10.1016/j.canep.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Zhao D, Jiang L, Hahn EW, Mason RP. Tumor physiologic response to combretastatin A4 phosphate assessed by MRI. International Journal of Radiation Oncology, Biology, Physics. 2005;62:872–880. doi: 10.1016/j.ijrobp.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Zhou F, Hu J, Shao JH, Zou SB, Shen SL, Luo ZQ. Metronomic chemotherapy in combination with antiangiogenic treatment induces mosaic vascular reduction and tumor growth inhibition in hepatocellular carcinoma xenografts. Journal of Cancer Research and Clinical Oncology. 2012;138:1879–1890. doi: 10.1007/s00432-012-1270-7. [DOI] [PubMed] [Google Scholar]