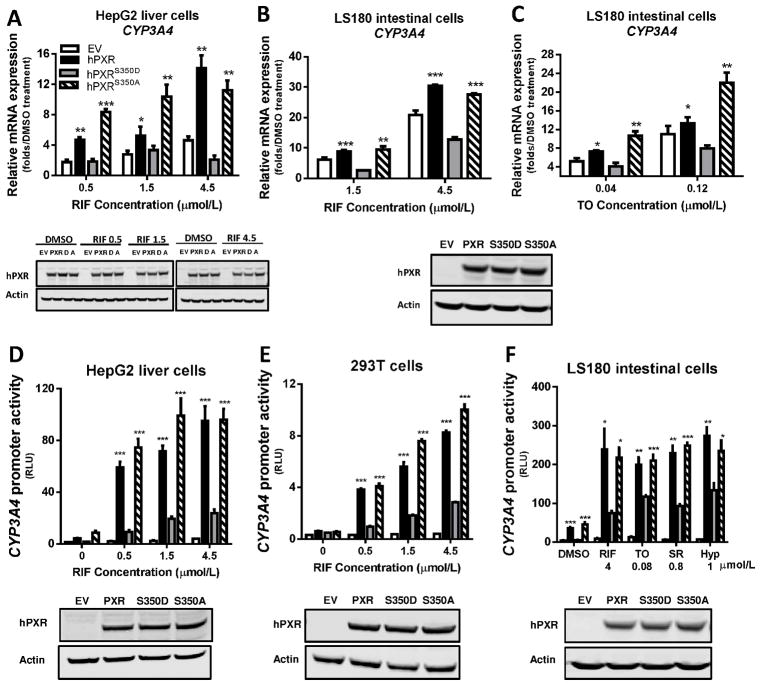
Figure 1. Attenuated transcriptional activation by hPXRS350D in human cells.
A–C) Human CYP3A4 mRNA level was quantified by real-time PCR in HepG2 liver cells and LS180 intestine cells transiently transfected for 24 h with pcDNA3 (empty vector, EV), pcDNA3-hPXR (hPXR), pcDNA3-hPXRS350D (hPXRS350D, or “D”), or pcDNA3-hPXRS350A (hPXRS350A, or “A”) and treated with 0.1% DMSO or increasing concentrations of rifampicin (RIF) or T0901317 (TO) as indicated for another 24 h. Results are presented as fold expression in DMSO-treated control cells. D-F) CYP3A4 promoter activity was determined in HepG2, 293T, and LS180 cells transiently co-transfected for 24 h with pGL3-CYP3A4-luc reporter and pRL-TK Renilla luciferase (Rluc, transfection control) and with empty vector, hPXR, hPXRS350D, or hPXRS350A plasmids and treated for another 24 h with 0.1% DMSO (“0” RIF), increasing concentrations of RIF (D and E), or different hPXR agonists (F). SR, SR12813, Hyp, hyperforin. CYP3A4 promoter activity is presented as relative luciferase units (RLU), normalized to Renilla luciferase. Data represent mean ± SEM from three independent experiments: *, P<0.05; **, P<0.01; ***, P<0.001 as compared by t test to cells expressing hPXRS350D mutant. The protein levels of hPXR, hPXRS350D (D) and hPXRS350A (A) in transfected cells were determined by Western blotting, and equal loading of lysates was verified by using Actin as control. The Western blots were placed under each bar graph (A, D, E and F); for B and C, only one Western blot showing the levels of PXR, S350D and S350A in LS180 cells was shown under the bar graphs.