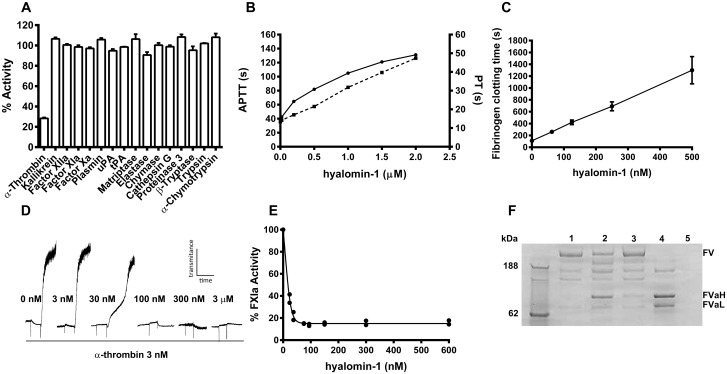
Fig 2. Hyalomin-1 is a specific thrombin inhibitor.
(A) The activity of 16 serine proteases in the presence of hyalomin-1 (1 μM) relative to their activity in the absence of inhibitor. (B) Coagulation time of human plasma incubated with hyalomin-1 as measured using the APTT (solid line), and PT (dashed line) assay procedures. (C) Conversion of fibrinogen to fibrin by thrombin in the presence of increasing concentrations of hyalomin-1 as indicated by seconds for increase in absorbance to 0.01 at 650 nm. (D) Aggregation of washed platelets induced by thrombin in the presence of various concentrations of hyalomin-1, as measured by an increase in transmittance in an aggregometer. (E) Polyphosphate-activated cleavage of FXI by thrombin in presence of hyalomin-1. FXIa was measured by hydrolysis of the chromogenic substrate S2236. (F) FV cleavage by thrombin in the presence and absence of hyalomin-1 as measured by SDS-PAGE. Lane 1 –FV alone after 60 min incubation. Lane 2 –FV and thrombin after 10 minutes incubation. Lane 3 –FV, thrombin and hyalomin-1 after 10 minutes incubation. Lane 4 –FV and thrombin after 60 minutes incubation. Lane 5 –thrombin alone.