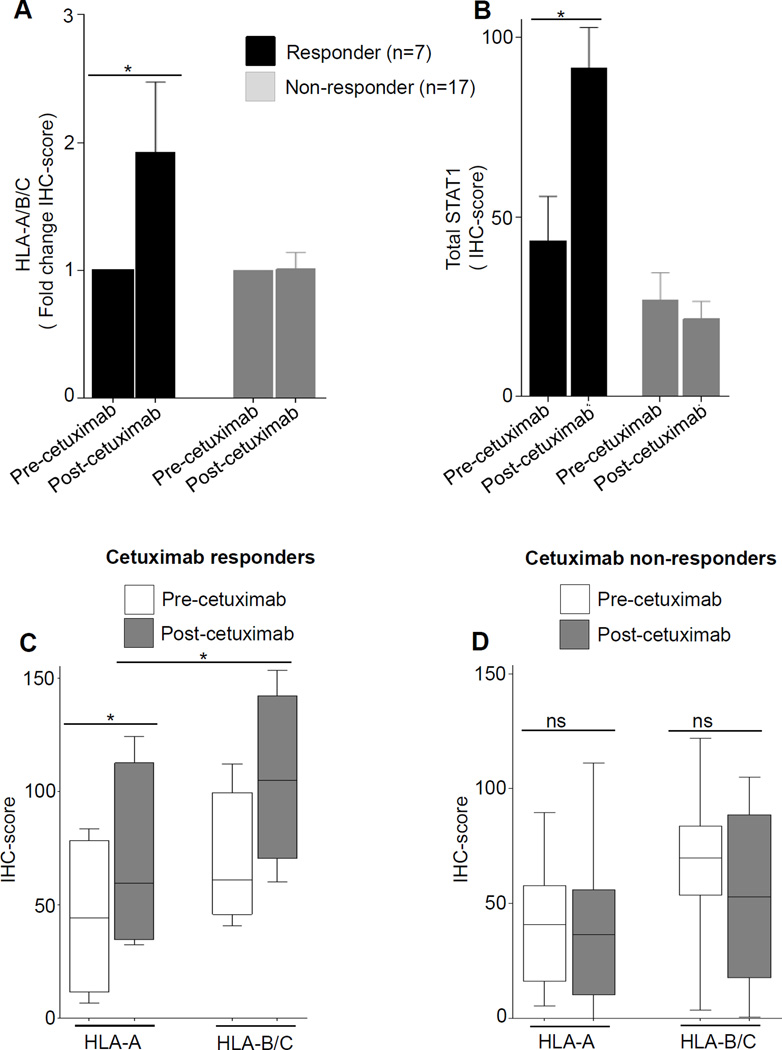
Figure 6. Cetuximab neoadjuvant therapy enhances HLA class I expression in patients’ tumors in clinical responder but not in nonresponder HNC patients.
Tumor specimens from HNC patients (n=24) enrolled on UPCI trial 08-013 were biopsied pre-treatment and post-neoadjuvant treatment (cetuximab IV 400mg/m2 day 1 then 250 mg/m2 alone days 8, 15, and 22), and a tissue microarray was prepared from paired tumor biopsies. Expression of HLA-A (mAb HCA2), HLA-B (mAb HC10), and STAT1 (mAb C-24) were determined in neoadjuvant cetuximab-treated HNC patients by semiquantitative IHC (A-D), and correlated with tumor shrinkage (CT scan “responders”) after 4 weeks of cetuximab therapy. (* P≤0.05)